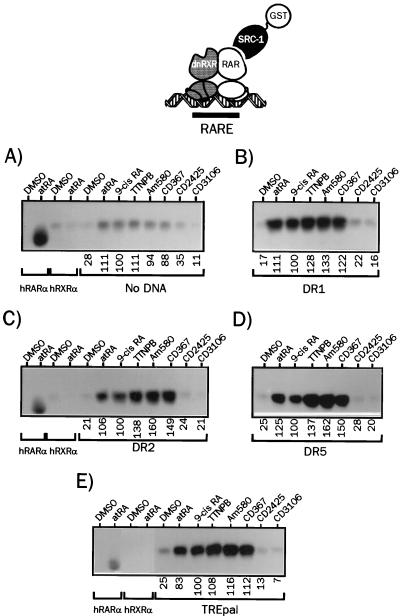
FIG. 7.
Deletion of RXR AF-2 abolishes selective retinoid-induced SRC-1 binding to hRXRα-hRARα heterodimers. (A) 35S-labeled dnRXRα and radioinert hRARα produced by coupled in vitro transcription-translation were incubated in the presence of the indicated retinoids at 10 μM (final concentration), and complexes were bound to a GST–SRC-1 fusion protein. After a 2-h incubation, complexes were isolated by adsorption on a glutathione-linked agarose matrix and analyzed by SDS-PAGE (8% gel). (B to E) dnRXRα-hRARα heterodimers associated to the indicated RARE were analyzed for the ability to interact with SRC-1 in the presence of natural or synthetic retinoids as described above. The nature of the response element is indicated below each panel. Numbers below gel lanes indicate the amount of radiolabeled receptor bound to SRC-1 in the presence of the indicated ligand relative to that measured in the presence of 9-cis RA, defined as 100%. Values represent mean data from four independent experiments carried out with two different bacterial extracts. Standard errors never exceeded 4.1%. Representative autoradiograms are shown.