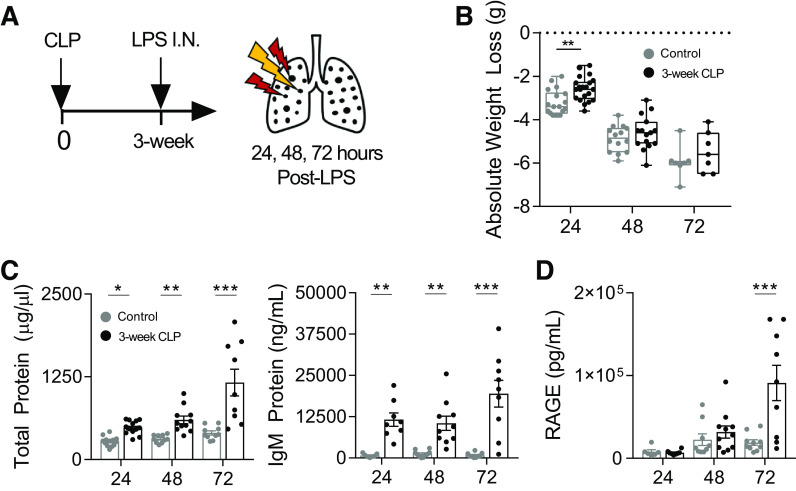
Figure 2.
LPS-induced lung injury is enhanced in mice surviving CLP. 3-wk post-CLP mice and age-matched unoperated controls underwent intranasal LPS (50 µg) administration. Lung injury was measured in BAL at 24, 48, and 72 h after administration (A). Weight loss trajectories after intranasal LPS in unoperated (24, 48, and 72 h post-LPS; n = 18, 14, 7, respectively) and 3 wk post-CLP (24, 48, and 72 h post-LPS; n = 21, 15, 7, respectively; box and whisker plot showing median, 25th percentile, 75th percentile, max and min; B). Lung permeability measured by total protein and IgM (C) and type I alveolar epithelial cell injury by soluble RAGE (D) in BAL by ELISA. Data represent two to three independent experiments per time point, total n = 7–21 mice per group. Mean ± SE shown. Two-way ANOVA all P < 0.01 for effect of CLP (B–D), post hoc Sidak (B) or Fisher’s least significant difference (C, D) P-values shown *P < 0.05, **P < 0.01, ***P < 0.001. BAL, bronchoalveolar lavage; CLP, cecal ligation and puncture; I.N., intranasal; LPS, lipopolysaccharide; RAGE, receptor for advanced glycation end-products.