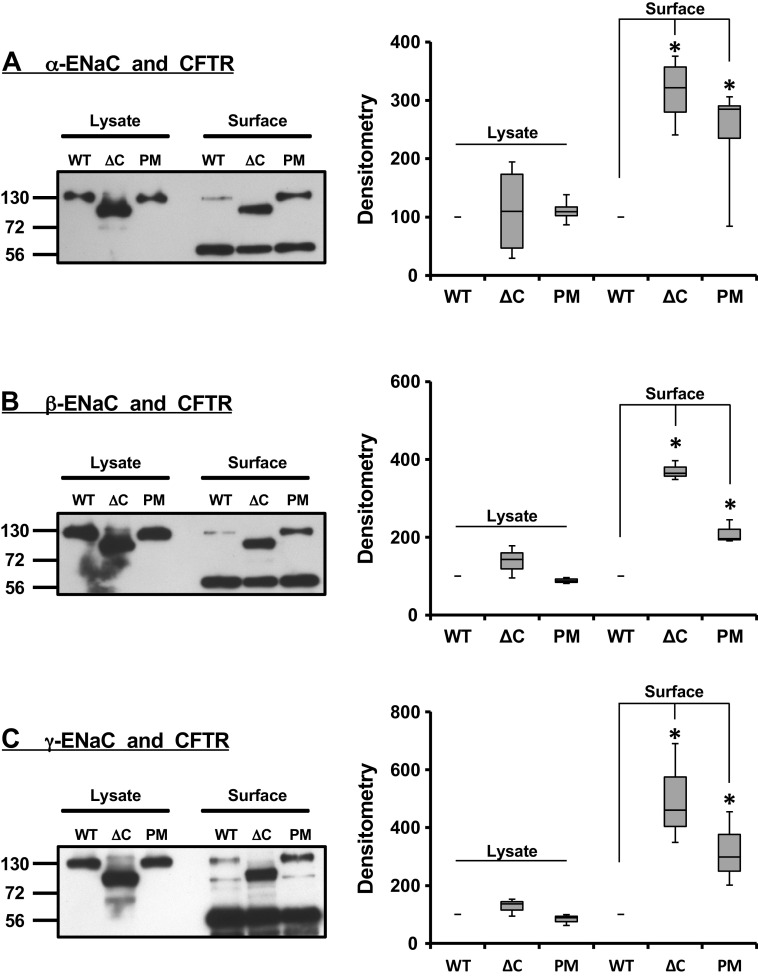
Figure 1.
Surface coimmunoprecipitation of mutated epithelial sodium channel (ENaC) subunits with cystic fibrosis transmembrane conductance regulator (CFTR). Surface biotinylation was combined with coimmunoprecipitation to monitor changes in surface expression of CFTR and α-ENaC (A), CFTR and β-ENaC (B), and CFTR and γ-ENaC (C). The cells were cotransfected with CFTR and all three ENaC subunits: in each case one of three subunits in the αβγ-ENaC complex was tagged with enhanced yellow fluorescent protein (EYFP). In all blots and graphs, the wild-type construct of ENaC subunit with EYFP at C-terminus is denoted as WT, the subunit truncated at C-terminus and N-terminally tagged with EYFP designated as ΔC, and the subunit with single-point mutation at C-terminus and C-terminally tagged with EYFP indicated as PM. Both the C-terminal truncation and single-point mutation at C-terminus of each individual ENaC subunit enhanced the coimmunoprecipitation signal and was statistically significant (*the corresponding densitometry bar graphs. Statistical significance was calculated using the Student’s t test). The changes in densitometry values following the C-terminal truncation and single point mutation were normalized to that of WT (100%). The bands at 56 kDa in each panel indicate the high-molecular weight immunoglobulin band. These experiments were repeated at least five times with similar results.