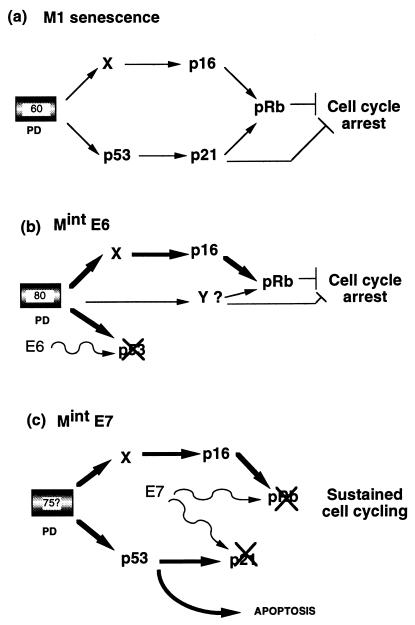
FIG. 9.
Models to explain Mint growth arrest states in human fibroblasts. (a) Normal senescence (M1) is assumed to be mediated by at least two signal pathways activated by a cell-cycle clock after around 60 PD (now almost certainly linked to telomere erosion). Both of these converge to inhibit phosphorylation of Rb by CDKs. p21 may also inhibit cell cycle progression via other targets (51). (b) MintE6. Cells which have initially escaped senescence by E6-mediated loss of the p53 pathway are able to reestablish cell cycle arrest approximately 20 PD later. One mechanism is further upregulation of the p16 pathway, which may compensate for the loss of p21, although our data do not exclude the requirement for an additional, as-yet-unknown, pathway(s) indicated by Y. (c) MintE7. Escape from senescence induced by HPVE7 is associated with a more profound disruption of cell cycle control through abrogation of pRb and p21 (22, 30a), which prevents restoration of cell cycle arrest. Clonal expansion is finally limited by apoptosis mediated at least in part by p53.