Abstract
Disseminated intravascular coagulation (DIC) is a rare paraneoplastic complication in advanced solid malignancies, with success of treatment and survival dependent on treatment of the underlying malignancy. Best estimates suggest an incidence of 1.6–6.8% in cancer, with risk factors being advanced disease, older age, and adenocarcinoma, especially of lung origin. Few cases, however, have reported on an association between DIC and oncogene‐addicted lung cancers, especially those containing ROS proto‐oncogene 1 (ROS1) mutations, however precedent exists to suggest increased prothrombotic rates in tumors harboring this mutation. We present a young woman with ROS1‐mutant non‐small‐cell lung cancer who presented in DIC and subsequently developed complications of both hemorrhage and thrombosis. Following initiation of targeted treatment, rapid resolution of laboratory coagulation derangement was observed and clinical improvement quickly followed. This event underscores the need for rapid evaluation of lung molecular panels and the dramatic resolution of life‐threatening illness that can occur with institution of appropriate therapy. This case contributes to growing evidence for a possible underlying link between oncogene addicted tumors and abnormalities of coagulation.
Keywords: disseminated intravascular coagulation, non‐small‐cell lung cancer, oncogene addiction, paraneoplastic syndrome, ROS1 mutation
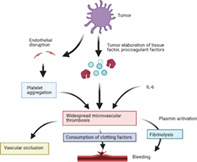
Suggested mechanism of action of tumor‐mediated disseminated intravascular coagulation (DIC).
INTRODUCTION
DIC (disseminated intravascular coagulopathy) is a clinicopathological syndrome characterized by consumptive coagulopathy without localization. One manifestation is as decompensated DIC, presenting with both bleeding and thrombosis with a predilection for microvasculature, but in solid malignancies such clinical presentations are often indolent and highly variable, depending on the efficacy of compensatory mechanisms and the intensity of the underlying malignancy.1, 2, 3 Few cases have reported on an association between DIC and oncogene‐addicted lung cancers. In this report, we present a young woman with ROS1‐mutant non‐small‐cell lung cancer who presented in DIC with excellent response to initiation of targeted treatment.
CASE PRESENTATION
A 54‐year‐old female ex‐light‐smoker with no significant comorbidities presented to the emergency department in February 2021 with symptoms of lethargy, headaches, and myalgias for 2 weeks. Outpatient imaging on the day of presentation with a CT chest, abdomen, and pelvis displayed stigmata of diffuse metastatic disease with innumerable pulmonary, intracranial, hepatic, and sclerotic osseous lesions (Figure 1(a)).
FIGURE 1.
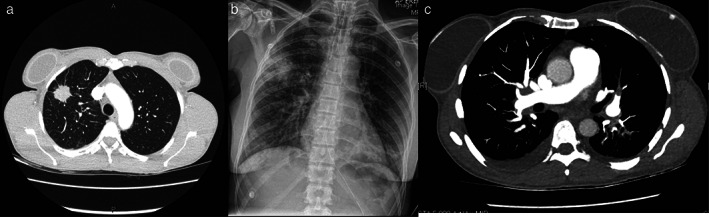
(a) Axial view from computed tomography of chest displaying primary lung lesion. (b) Plain film x‐ray of perilesional hematoma with associated haemothorax. (c) Computed tomography axial view of segmental pulmonary emboli
Thorough physical examination was unremarkable. She was febrile on admission and pathology results noted an elevated bilirubin at 25 μmol/L, moderately deranged liver enzymes in a cholestatic pattern, and mildly abnormal coagulation profile with international normalized ratio (INR) 1.6 IU, activated partial thromboplastin time (APTT) 40.5 s, and prothrombin time (PT) 21.4 s. Platelet count was 258 × 109/L. She was commenced on IV vitamin K in the context of liver dysfunction and lung biopsy confirmed primary lung adenocarcinoma. Shortly after biopsy she developed a moderate perilesional hematoma at the site of biopsy and a small hemothorax (Figure 1(b)), requiring intubation and respiratory support. She improved with supportive measures and was extubated after 24 h. Worsening coagulation derangement was noted, hematology was consulted, and the diagnosis of diffuse intravascular coagulopathy (DIC) was confirmed.
She was commenced on intensive blood product support, eventually comprising 9 units of packed red blood cells, 70 units of cryoprecipitate, and 6 units of fresh frozen plasma, aiming for a fibrinogen level >1.5 g/L, with discussions commenced about emergent administration of chemotherapy with carboplatin and pemetrexed at day 9 of her admission. During this time emergency radiation was also administered (12Gy/2#) for both intracranial and pulmonary lesions due to persistent hemoptysis with dosing selected to avoid overlap with her anticipated systemic therapy.
Shortly after her first cycle of chemotherapy, immunohistochemistry (IHC) on her biopsy sample showed strong diffuse staining for ROS proto‐oncogene 1 (ROS1) protein (SP384 antibody). Epidermal growth factor receptor (EGFR) testing and anaplastic lymphoma kinase (ALK) IHC were negative. Fluorescence in situ hybridization (FISH) confirmed a ROS1 rearrangement (Figure S1), and the decision was made to switch treatment to entrectinib.
Prior to this, she suffered a further desaturation event with cyanosis, chest pain, and tightness. CT pulmonary angiography confirmed bilateral segmental and subsegmental pulmonary emboli with imaging evidence of right heart strain, and she again required intensive care admission for respiratory support, heparin infusion, and ongoing management of DIC (Figure 1(c)).
Following institutional approval, entrectinib was commenced 23 days after diagnosis. Dramatic laboratory improvement of DIC followed within 3 days with no further blood product requirements (Figures S2 and S3). She was discharged 7 days later.
At subsequent outpatient follow‐up she remained well with no further evidence of DIC and near normalization of liver function. Initial progress imaging after 6 weeks noted a partial response, and she continues on entrectinib with good tolerance.
DISCUSSION
DIC is a rare complication of advanced malignancy, with the best estimation of incidence as 6.8% from one large cohort study. This study noted the diagnosis to be most common in adenocarcinomas of lung, breast, prostate, colorectal, and pancreatic origin.4 Multivariate analysis in this cohort also suggested an increased risk for older patients, male gender, advanced malignancies, and presence of necrosis in the tumor specimen.4 In contrast to this, Kanaji et al. assessed 716 patients with pathologically proven lung cancer and reported a prevalence of DIC of only 0.7% overall and 1.5% in stage IV disease.5
Besides these studies, reports are otherwise limited to isolated cases, and despite the low prevalence suggested by Kanaji et al., non‐small‐cell lung cancers (NSCLC) predominate. Most of these cases pre‐date widespread use of methods to detect molecular fusions and therefore whether an association exists between oncogene driver mutations and DIC is uncertain. The few cases in which an oncogene driver was demonstrated are given in Table 1. Of note, however, ROS1‐rearranged NSCLCs have been associated with a higher incidence of venous thromboembolism. Recent analysis from the METROS trial noted a 3–5‐fold higher incidence of venous thromboembolism in ROS1‐rearranged NSCLC compared to ROS1 wild‐type NSCLCs.6
TABLE 1.
Table of reported cases of disseminated intravascular coagulation in lung cancers with driver mutations
| Author | Year | Study | Number | Mutation | Stage | Comment |
|---|---|---|---|---|---|---|
| Brosseau et al.15 | 2018 | Case report | 1 | ROS1 | IV | Complete response to crizotinib |
| Kim et al.16 | 2018 | Case report | 1 | EGFR | IV | |
| Fujita et al.17 | 2021 | Case report | 1 |
EGFR ALK |
IV |
L858R Treated successfully with osimertinib |
| Toyokawa et al.18 | 2013 | Case report | 1 | ALK | IV | |
| De Giglio et al.19 | 2017 | Case report | 1 | ALK | IV | Died before treatment institution |
| Tanaka et al.20 | 2016 | Case report | 1 | ALK | IV | Rapid response to alectinib |
In the setting of malignancy, DIC is posited to result from endothelial disruption compounded by tumor elaboration of procoagulant molecules, such as tissue factor (TF) and cancer procoagulant, driven by pro‐inflammatory cytokines, especially interleukin‐6.7, 8 This results in platelet aggregation with widespread microvascular thrombosis, consumption of coagulation factors, and generation of fibrin degradation products due to compensatory fibrinolysis (Figure 2). Success of DIC treatment therefore revolves around treatment of the underlying malignant process,1 confirmed in this case with dramatic resolution of requirements for blood products and no further thrombotic or hemorrhagic events following commencement of entrectinib. This event underscores the importance of rapid evaluation of molecular panels in the setting of newly diagnosed metastatic lung cancer. Initial treatment with carboplatin and pemetrexed was rationalized in the context of a life‐threatening hemorrhagic event, although following ROS1 positivity treatment was swiftly changed due to greater proven efficacy with targeted treatments compared to chemotherapy.9
FIGURE 2.
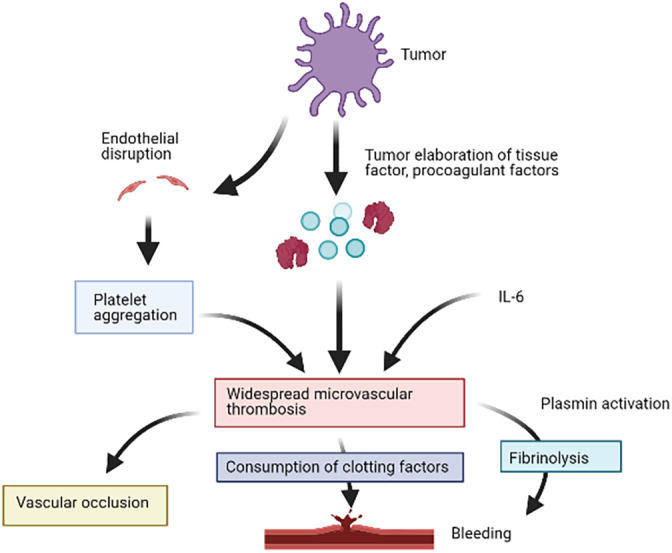
Suggested mechanism of action of tumor‐mediated disseminated intravascular coagulation
ROS1 fusions occur in roughly 1–2% of NSCLC, typically in adenocarcinomas in light or nonsmokers. These are oncogenic drivers with constitutive tyrosine kinase activity activating cell survival and growth signaling pathways, including MAPK/ERK, JAK/STAT, and PI3K/AKT cascades.10 There is considerable partner gene heterogeneity with the most common ROS1 fusion in NSCLC being CD74‐ROS1, which is also associated with a higher incidence of intracranial metastases.11 ROS1 fusions are druggable targets in NSCLC, with a number of effective therapeutics.12 Entrectinib is a multitargeted tyrosine kinase inhibitor of ROS1 fusions, as well as Tropomyosin receptor kinase (TRK) A/B/C fusions and ALK, specifically designed for blood–brain barrier penetrance, making it the preferred choice of treatment in this case.13 Reported objective response rates in patients with intracranial disease exceed 70%, with a duration of response over 12 months.13 For this patient ongoing follow‐up will be required to monitor closely for toxicities and early signs of disease progression.
In conclusion, this report describes a rare case of widespread de novo metastatic ROS1‐fusion positive lung cancer with an initial presentation of DIC causing numerous complications, which promptly resolved following commencement of targeted therapy. However, despite high initial responses, resistance to targeted treatments is common, and it remains uncertain whether DIC could re‐manifest as a sign of development of resistance.14
CONFLICT OF INTEREST
The authors have no competing interests to declare.
Supporting information
Supporting Information Figure S1 ROS1 FISH using a breakapart probe showing a dominant pattern of split red and green signals and single green signals in keeping with ROS1 rearrangement
Supporting Information Figure S2 Longitudinal graph of coagulation values, arrow indicating commencement of targeted treatment
Supporting Information Figure S3 Timeline from presentation with complications and treatments
ACKNOWLEDGMENTS
The authors have no additional acknowledgements.
Woodford R, Lu M, Beydoun N, Cooper W, Liu Q, Lynch J, et al. Disseminated intravascular coagulation complicating diagnosis of ROS1‐mutant non‐small cell lung cancer: A case report and literature review. Thorac Cancer. 2021;12:2400–2403. 10.1111/1759-7714.14071
REFERENCES
- 1.Levi M, ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–92. [DOI] [PubMed] [Google Scholar]
- 2.Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30. [PubMed] [Google Scholar]
- 3.Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol. 2021;192:803–18. [DOI] [PubMed] [Google Scholar]
- 4.Sallah S, Wan JY, Nguyen NP, Hanrahan LR, Sigounas G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001;86:828–33. [PubMed] [Google Scholar]
- 5.Kanaji N, Mizoguchi H, Inoue T, Tadokoro A, Watanabe N, Ishii T, et al. Clinical features of patients with lung cancer accompanied by thromboembolism or disseminated intravascular coagulation. Ther Clin Risk Manag. 2018;14:1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiari R, Ricciuti B, Landi L, Morelli AM, Delmonte A, Spitaleri G, et al. ROS1‐rearranged non–small‐cell lung cancer is associated with a high rate of venous thromboembolism: analysis from a phase II, prospective, multicenter, two‐arms trial (METROS). Clin Lung Cancer. 2020;21:15–20. [DOI] [PubMed] [Google Scholar]
- 7.Sato T, Tsujino I, Ikeda D, Ieko M, Nishimura M. Trousseau's syndrome associated with tissue factor produced by pulmonary adenocarcinoma. Thorax. 2006;61:1009–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levi M. Clinical characteristics of disseminated intravascular coagulation in patients with solid and hematological cancers. Thromb Res. 2018;164:S77–81. [DOI] [PubMed] [Google Scholar]
- 9.Gridelli C, De Marinis F, Di Maio M, Cortinovis D, Cappuzzo F, Mok T. Gefitinib as first‐line treatment for patients with advanced non‐small‐cell lung cancer with activating epidermal growth factor receptor mutation: review of the evidence. Lung Cancer. 2011;71:249–57. [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo A, Sobhani N, Chapman R, Bagby S, Bortoletti C, Traversini M, et al. Focus on ROS1‐positive non‐small cell lung cancer (NSCLC): crizotinib, resistance mechanisms and the newer generation of targeted therapies. Cancer. 2020;12:3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drilon A, Jenkins C, Iyer S, Schoenfeld A, Keddy C, Davare MA. ROS1‐dependent cancers – biology, diagnostics and therapeutics. Nat Rev Clin Oncol. 2021;18:35–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon BJ, Loong HHF, Summers YJ, Thomas ZM, French PP, Lin BK, et al. Correlation between overall response rate and progression‐free survival/overall survival in comparative trials involving targeted therapies in molecularly enriched populations. J Clin Oncol. 2020;38(15 Suppl):3588. [Google Scholar]
- 13.Drilon A, Siena S, Dziadziuszko R, Barlesi F, Krebs MG, Shaw AT, et al. Entrectinib in ROS1 fusion‐positive non‐small‐cell lung cancer: integrated analysis of three phase 1‐2 trials. Lancet Oncol. 2020;21:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voulgaris E, Pentheroudakis G, Vassou A, Pavlidis N. Disseminated intravascular coagulation (DIC) and non‐small cell lung cancer (NSCLC): report of a case and review of the literature. Lung Cancer. 2009;64:247–9. [DOI] [PubMed] [Google Scholar]
- 15.Brosseau S, Gounant V, Naltet C, Théou‐Anton N, Cazes A, Smonig R, et al. Lazarus syndrome with crizotinib in a non‐small cell lung cancer patient with ROS1 rearrangement and disseminated intravascular coagulation. Clin Lung Cancer. 2018;19:e57–61. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Ryu JS, Jeon SH, Kim HJ, Nam HS, Cho JH, et al. Dramatic response of acute disseminated intravascular coagulation to erlotinib in a patient with lung adenocarcinoma with activating EGFR mutation. J Int Med Res. 2018;46:533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita K, Naka M, Ito T, Kanai O, Maekawa K, Nakatani K, et al. Successful management of a lung cancer patient harbouring both EGFR mutation and EML4‐ALK fusion gene with disseminated intravascular coagulation. Respir Med Case Rep. 2021;33:101393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toyokawa G, Takenoyama M, Watanabe S, Toyozawa R, Inamasu E, Kojo M, et al. Dramatic response to crizotinib in an ALK‐positive adenocarcinoma patient with disseminated intravascular coagulation. J Thorac Oncol. 2013;8:e96–8. [DOI] [PubMed] [Google Scholar]
- 19.De Giglio A, Porreca R, Brambilla M, Metro G, Prosperi E, Bellezza G, et al. Fatal acute disseminated intravascular coagulation as presentation of advanced ALK−positive non‐small cell lung cancer: does oncogene addiction matter? Thromb Res. 2018;163:51–3. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Taima K, Morimoto T, Nakamura K, Tanaka Y, Itoga M, et al. Dramatic response to alectinib in a patient of ALK‐rearranged lung cancer with poor performance status. BMC Res Notes [Internet]. 2016; 9:173. http://europepmc.org/abstract/MED/26987388; 10.1186/s13104-016-1983-9; https://europepmc.org/articles/PMC4794901; https://europepmc.org/articles/PMC4794901?pdf=render. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1 ROS1 FISH using a breakapart probe showing a dominant pattern of split red and green signals and single green signals in keeping with ROS1 rearrangement
Supporting Information Figure S2 Longitudinal graph of coagulation values, arrow indicating commencement of targeted treatment
Supporting Information Figure S3 Timeline from presentation with complications and treatments


