FIGURE 1.
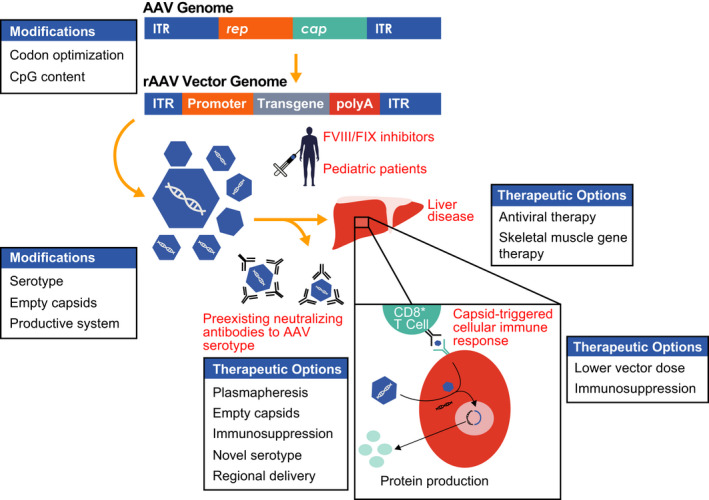
Overview of rAAV‐mediated liver‐directed gene therapy for hemophilia. The wild‐type adeno‐associated virus (AAV) genome consists of two inverted tandem repeat (ITR) regions flanking the rep (replication) and cap (capsid) genes. These genes are replaced by a tissue‐specific promoter with enhancer, intron, and transgene of interest in the recombinant adeno‐associated viral (rAAV) vector transgene expression cassette, which is packaged into capsids and injected into subjects via an intravenous infusion. Once infused, rAAV vector can be neutralized by preexisting antibodies in a serotype‐specific manner or transduce hepatocytes. The capsid is degraded and the genetic material maintained as an episome in the nucleus to produce the transgene product. Capsid peptides can be presented on the surface of hepatocytes to CD8+ T cells, thought to lead to a cellular immune response coinciding with loss of transgene and a rise in liver transaminases in some clinical trials. Modifications in the transgene, serotype, infusion of empty capsids, and production process may all affect efficacy. Options to bypass the preexisting humoral response or liver disease are listed. Additional hurdles to general application of liver‐directed AAV gene therapy include inhibitors to factor VIII (FVIII) and factor IX (FIX) as well as infusion in young people with hemophilia. (Reproduced, with permission, from Doshi et al, p 275, Figure 1)
