Abstract
Asthma exacerbations are a major cause of intractable morbidity, increases in health care costs, and a greater progressive loss of lung function. Asthma exacerbations are most commonly triggered by respiratory viral infections, particularly with human rhinovirus (hRV). Respiratory viral infections are believed to affect the expression of indoleamine 2,3-dioxygenase (IDO), a limiting enzyme in tryptophan catabolism, which is presumed to alter asthmatic airway inflammation. Here, we explored the detailed role of IDO in the progression of asthma exacerbations using a mouse model for asthma exacerbation caused by hRV infection. Our results reveal that IDO is required to prevent neutrophilic inflammation in the course of asthma exacerbation caused by an hRV infection, as corroborated by markedly enhanced Th17- and Th1-type neutrophilia in the airways of IDO-deficient mice. This neutrophilia was closely associated with disrupted expression of tight junctions and enhanced expression of inflammasome-related molecules and mucin-inducing genes. In addition, IDO ablation enhanced allergen-specific Th17- and Th1-biased CD4+ T-cell responses following hRV infection. The role of IDO in attenuating Th17- and Th1-type neutrophilic airway inflammation became more apparent in chronic asthma exacerbations after repeated allergen exposures and hRV infections. Furthermore, IDO enzymatic induction in leukocytes derived from the hematopoietic stem cell (HSC) lineage appeared to play a dominant role in attenuating Th17- and Th1-type neutrophilic inflammation in the airway following hRV infection. Therefore, IDO activity in HSC-derived leukocytes is required to regulate Th17- and Th1-type neutrophilic inflammation in the airway during asthma exacerbations caused by hRV infections.
Keywords: Indoleamine 2,3-dioxygenase; Rhinovirus; Asthma exacerbation; Th17 inflammation; Hematopoietic stem cells
INTRODUCTION
Asthma is intractable airway inflammation that is globally associated with significant morbidity, hospitalization, and death. Asthmatic airway inflammation is defined as a heterogeneous disease that affects 334 million people worldwide, up to 10% of adults and 30% of children, and its incidence is expected to increase to around 400 million people by 2025 (1). The heterogeneity of asthmatic airway inflammation involves multifaceted interactions among many risk factors and disease determinants (1). To date, the canonical phenotypes of asthmatic airway inflammation are well defined by Th2 type–mediated signatures: eosinophil infiltration, excessive secretion of mucus, allergen-specific Th2 responses, and airway remodeling following aero-allergen sensitization (2).
However, irrespective of asthma severity and despite optimal medical therapy, asthmatic patients often experience acute exacerbations of symptoms and a loss of disease control. Asthma exacerbations are a major cause of disease morbidity, increases in health care costs, and a greater progressive loss of lung function (3,4,5). The frequency and severity of asthma exacerbations can be reduced, but not always fully prevented, by using an inhaled corticosteroid (ICS) or combination ICS/long-acting β-agonist (5). It is currently believed that asthma exacerbations are most commonly triggered by viral respiratory infections, particularly with human rhinovirus (hRV). Allergic sensitization can be a risk factor for wheezing with hRV infection, particularly in children (3,4,5,6). Whether the Th2-mediated airway inflammation found with allergen sensitization increases susceptibility to hRV infection or enhances its ability to provoke further inflammation is unclear, but evidence suggests that the type I IFN responses induced by viral infection are lower than normal in peripheral blood mononuclear cells (7,8,9), plasmacytoid dendritic cells (pDCs) (10), and bronchial epithelial cells (11,12) derived from patients with allergic asthma. In addition, the occupancy of FcεR with IgE was found to inhibit the antiviral generation of IFN-α from pDCs and could increase susceptibility to hRV-induced wheezing and asthma exacerbation (10). Thus, asthmatic patients with Th2-type inflammation are thought to harbor a defect in the type I IFN antiviral immune response to hRV infection, which could be inversely correlated with increasing airway eosinophilia, Th2-type cytokines, and serum IgE (12). Multiple studies have demonstrated a positive correlation between inflammatory cytokines and symptom severity following hRV infection. In particular, the production of CXCL8 (IL-8) and CXCL10 (IP-10), which lead to neutrophilic inflammatory responses, increased upon experimental hRV challenge in adults with mild asthma (13,14). A recent study reported that hRV infection induced an amplified antiviral Th1 response in asthmatic patients compared with controls, along with synchronized allergen-specific Th2 expansion (15), suggesting that hRV infection induces robust Th1 responses in allergic asthmatic subjects, which could promote disease. The dysfunctional regulation of immune cells in asthmatic patients following hRV infection contributes to severe clinical complications such as asthma exacerbation and chronic obstructive pulmonary disease, which can be life-threatening conditions (16). Additional mechanisms for hRV-induced asthma exacerbation have been suggested, including an increase in epithelial cells producing IL-25 and IL-33, which promote Th2-type inflammation (17,18), and enhanced expression of the hRV receptor CDHR3 in the airway epithelium (19,20). Elucidating the complex links between immune cells and related molecules will clarify the mechanisms of hRV-induced asthma exacerbation.
Indolamine 2,3-dioxygenase (IDO), a rate-limiting enzyme in the catabolism of tryptophan (TRP) to kynurenine (KYN) (KYN-IDO pathway), has long been acknowledged to contribute substantially to the control of general inflammation (21,22,23). The immunomodulatory function of TRP metabolism has been increasingly demonstrated in infection, pregnancy, autoimmunity, and neoplasia (21,22,23). One area of particular interest in studying the KYN-IDO pathway is its role in the control and development of allergic disease via the resultant depletion of serum TRP and increase in TRP metabolites (24,25). IDO induction in the KYN-IDO pathway almost always leads to preferential apoptosis of Th1 cells, and IDO plays a role in regulating the immune system by reducing Th1 cell proliferation and inducing the differentiation of Treg cells (26,27,28), thereby maintaining immunological tolerance and limiting tissue damage. Compared with the inhibitory role of IDO in Th1 cell differentiation, the outcome from inducing IDO on Th2 cells is more complex, with both inhibitory and stimulatory actions reported (29). IDO-expressing human eosinophils and the addition of exogenous TRP induced a preferential decrease in Th1-type responses and consequent increase in Th2 cytokine production (30). Unlike reports showing Th2 cell promotion and Th1 inhibition, in vivo studies on allergy have shown contradictory effects for IDO. Compared with wild-type (WT) mice, IDO-deficient mice displayed significantly weaker Th2 responses and lower levels of allergen-specific IgE in sera when challenged with an inhaled Ag, suggesting that IDO ablation results in a protective effect against allergic disease (31). In another murine model of asthma using ovalbumin (OVA) sensitization, inducing IDO expression with an IDO-inducing molecule such as a TLR9 agonist inhibited Th2-type airway inflammation in the lung, and IDO expression by resident lung tissue cells seemed more instrumental than the activity of dendritic cells (DCs) or macrophages (32). Only a few studies have suggested that IDO has a role in the control of inflammation in asthmatic patients. In the sera of patients with allergic asthma, the ratio of KYN to TRP was lower than in a control group of non-asthmatic individuals, which suggests lower IDO activity in allergic patients (33). Also, low IDO activity is increased by inhaled steroid treatment in patients with mild to moderate persistent asthma (34), and a significant correlation was observed between IDO and Th17/Treg imbalance in children with allergic asthma (35). Therefore, inducing the KYN-IDO pathway and lowering the systemic TRP concentration could help to control asthmatic diseases.
Respiratory viral infection can affect IDO expression and thereby alter asthmatic airway inflammation. Respiratory syncytial virus (RSV), another important cause of asthma exacerbation, induces the expression and bioactivity of IDO in human DCs derived from monocytes, which suggests that IDO has a potentially novel role in the development of asthma exacerbation (36). In contrast, RSV reduces KYN production and reverses the Th17/Treg balance by modulating IDO expression in pDCs (36). Also, infection with the influenza A virus attenuates IDO expression and the production of KYN in lung tissues (37). Furthermore, systemic TRP and KYN levels were closely related to the severity of hRV-induced asthma exacerbation in an experimental patient model (38). Although IDO activity was not induced by hRV infection due to the very low infection dose, systemic TRP and its catabolites were markedly higher in patients with allergic asthma. Thus, the enhanced systemic catabolism of TRP in patients with allergic asthma could be strongly related to the outcome of hRV-induced asthma exacerbation. Despite those previous results, little is known about the role of IDO in asthma exacerbations caused by hRV infections in terms of results from a practical infection model. In this work, we explored the precise role of IDO in the progression of asthma exacerbations by using a mouse model for asthma exacerbation caused by hRV infection. Our results reveal that IDO deficiency greatly increases neutrophil infiltration in the airways during acute and chronic asthma exacerbations caused by hRV. The asthma exacerbation manifested as neutrophilic airway inflammation was mediated by markedly enhanced Th17- and Th1-type responses in IDO-ablated mice. Furthermore, IDO deficiency in leukocytes derived from the hematopoietic stem cell (HSC) lineage was found to play a major role in inducing neutrophilic airway inflammation mediated by Th17- and Th1-type responses during the progression of hRV-induced asthma exacerbations. Therefore, our results suggest that IDO in HSC-derived leukocytes is needed to regulate neutrophilic airway inflammation in the course of hRV-induced asthma exacerbations.
MATERIALS AND METHODS
Ethics statement
All animal experiments described in this study were conducted at Jeonbuk National University, followed the guidelines of the Institutional Animal Care and Use Committee of Jeonbuk National University, and were pre-approved by the Ethics Committee for Animal Experiments at Jeonbuk National University (approval No. JBNU-2019-00079). The animal research protocol used in this study followed the guidelines set by the nationally recognized Korean Association for Laboratory Animal Sciences. All experimental protocols requiring biosafety were approved by the Institutional Biosafety Committee of Jeonbuk National University.
Animals, cells, and viruses
C57BL/6 (BL/6, H-2b) mice (6–8 weeks-old female or male) were purchased from Samtako (Osan, Korea). IDO-deficient (IDO KO) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). All mice were genotyped and bred in the animal facilities of Jeonbuk National University. Minor-group hRV 1B was obtained from the American Type Culture Collection (VR1645; Middlesex, UK) and passaged 6 times in the Ohio-HeLa human epithelial cell line (ECACC84121901; European Collection of Authenticated Cell Cultures, Salisbury, UK) before purification. Ohio-HeLa cells grown to around 90% confluency were infected with hRV-1B in DMEM supplemented with 2% FBS, penicillin (100 U/mL), and streptomycin (100 U/mL). Six to seven days post-infection (dpi), cultures of Ohio-HeLa cells showing an 80%–90% cytopathic effect were harvested. The virus was then concentrated through ultracentrifugation using 30% sucrose cushions, and viral stock was stored at −80°C until use in vivo. The hRV 1B was titrated on the Ohio-HeLa cells using standard methods for cytopathic effect.
Abs and reagents
The mAbs used for flow cytometric analysis and other experiments were obtained from eBioscience (San Diego, CA, USA) or BD Biosciences (San Diego, CA, USA): FITC-labeled anti-mouse CD4 (RM4-5), CD45 (30-F11); PE-labeled anti-mouse IFN-γ (XMG1.2), FoxP3 (FJK-16s); PerCP-labeled anti-mouse CD4 (L3T4, RM4-5); allophycocyanin (APC)-conjugated anti-mouse IL-17A (eBio17B7); biotin-conjugated anti-mouse IL-4 (BVD6-24G2), and streptavidin-conjugated PerCP. PMA and ionomycin were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Abs used to detect OVA-specific-IgE, IgG, IgG1, and IgG2a levels in sera were purchased from Southern Biotech (Birmingham, AL, USA): unconjugated (human absorbed) anti-mouse IgG, goat anti-mouse IgG1, goat anti-mouse IgG2a, goat anti-mouse IgE, HRP-conjugated goat anti-mouse IgG, goat anti-mouse IgG1, goat anti-mouse IgG2a, and rat anti-mouse IgE-HRP. The primers specific for cytokines, mucins, and tight junctions (TJs) were synthesized by Bioneer Corp. (Daejeon, Korea) and used for PCR amplification of target genes.
Mouse model of acute and chronic asthma exacerbations by hRV infection
Mouse models of asthma exacerbations caused by hRV infection were established as described elsewhere, with some modification (39). The experimental WT BL/6 and IDO KO mice were sensitized with 100 μg of chicken OVA (Grade V; Sigma-Aldrich) absorbed to 2 mg of aluminum hydroxide (alum; Sigma-Aldrich) in a volume of 200 μl of PBS via intraperitoneal (i.p.) injection. After 10 days, the mice were challenged with 50 μg of OVA (Sigma-Aldrich) in 30 μl of PBS (controls received PBS alone) on 3 consecutive days via the intranasal (i.n.) route under light anesthesia using isoflurane. For asthma exacerbation by hRV infection, the third OVA solution included hRV (1×107 TCID50). UV-inactivated hRV (1,200 mJ/cm2, 30 min) was used as the negative control for hRV. To establish a chronic model of hRV-induced asthma exacerbation, BL/6 and IDO KO mice were sensitized twice with 50 μg of OVA in 2 mg of alum on days 0 and 7 through i.p. injection. Ten days later, the mice were challenged with either 50 μg of OVA or PBS for 3 consecutive days via the i.n. route. For the hRV-induced asthma exacerbation in the chronic model, the third OVA solution included hRV (1.0×107 TCID50), and that 3-consecutive-days challenge was repeated 5 times every 7 days.
Quantitative real-time RT-PCR to determine cytokine, mucin, and TJ expression
The mRNA expression of inflammatory cytokines (Tnfa, Il6, Il13, Il17a, Cxcr2, myeloperoxidase (Mpo), Il25, Il33, and Il1b), mucus production–related genes (Muc5ac, Muc5b, and Gob5), TJ molecules (Zo1, claudin4, claudin5, and Jam), and inflammasome-related genes (Nlrp3, Nlrc4, and Asc) were assessed by real-time qRT-PCR using total RNA extracted from collected lung tissue. Total RNA was extracted from the collected lung tissues using easy-BLUE (iNtRON, Inc., Daejeon, Korea) and subjected to real-time qRT-PCR in a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Following reverse transcription of the total RNA with High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster, CA, USA), the reaction mixture (20 μl total) contained 2 μl of template cDNA, 10 μl of 2× SYBR Premix Ex Taq (WizBio Solutions, Jinju, Korea), and 200 nM primers (Table 1). The reactions were denatured at 95°C for 30 s and then subjected to 45 cycles of 95°C for 5 s and 60°C for 20 s. After completion of the reaction cycle, the temperature was increased from 65°C to 95°C at 0.2°C/15 s, and fluorescence was measured every 5 s to construct a melting curve. A control sample lacking the template DNA was run with each assay. All measurements were performed at least in duplicate to ensure reproducibility. The authenticity of the amplified product was determined using a melting curve analysis. The expression of cytokines and chemokines was normalized to the level of the housekeeping gene Gapdh. All data were analyzed using Bio-Rad CFX Manager, version 2.1 software (Bio-Rad Laboratories).
Table 1. Specific primers for the expression of different cytokines, mucins, inflammasomes, and TJs in quantitative RT-PCR.
| Gene name | Primer sequence (5′-3′) | Position cDNA | Gene bank ID |
|---|---|---|---|
| Il1b | F: AAG TGA TAT TCT CCA TGA GCT TTG T | 554-578 | NM_008361.4 |
| R: TTC TTC TTT GGG TAT TGC TTG G | 698-719 | ||
| Il6 | F: TGG GAA ATC GTG GAA ATG AG | 256-275 | NM_031168.2 |
| R: CTC TGA AGG ACT CTG GCT TTG | 489-509 | ||
| Il17a | F: TCT GAT GCT GTT GCT GCT G | 87-105 | XM_021176489.1 |
| R: ACG GTT GAG GTA GTC TGA GG | 254-273 | ||
| Il25 | F: ACA GGG ACT TGA ATC GGG TC | 336-355 | NM_080729.3 |
| R: TGG TAA AGT GGG ACG GAG TTG | 436-456 | ||
| Cxcr2 | F: CAT CTT ATA CAA CCG GAG CA | 213-232 | XM_021176908.1 |
| R: TAG TAA CCA CAT GGC TAT GC | 494-513 | ||
| Muc5ac | F: TGT GCC TGC CTG TAC AAT GG | 1220-1239 | NM_010844.3 |
| R: CCA GAA CAT GTG TTG GTG CAG | 1276-1296 | ||
| Muc5b | F: ATG GGC AGC AGA AAC TGG AG | 46-65 | NM_028801.2 |
| R: ATG GAG TCA CTA TAC ACT CTC TGA C | 170-194 | ||
| Gob5 | F: AAC GGA GCA GGT GCC GAT GC | 1967-1986 | NM_017474.2 |
| R: AGT GAC TCC TCC CAG AGC CCA | 2060-2080 | ||
| Zo1 | F: AGG ACA CCA AAG CAT GTG AG | 6227-6246 | NM_009386.2 |
| R: GGC ATT CCT GCT GGT TAC A | 6295-6313 | ||
| Claudin4 | F: GGG AAT CTC CTT GGC AGT CC | 216-235 | NM_009903.2 |
| R: CGA TGT TGC TGC CGA TGA AG | 291-310 | ||
| Claudin5 | F: GTG GAA CGC TCA GAT TTC AT | 1054-1073 | NM_013805.4 |
| R: TGG ACA TTA AGG CAG CAT CT | 1131-1150 | ||
| Jam | F: ACC CTC CCT CCT TTC CTT AC | 1136-1182 | NM_172647.2 |
| R: CTA GGA CTC TTG CCC AAT CC | 1238-1257 | ||
| Mpo | F: CCA GCA GCC ATG AAG AAG TA | 1621-1640 | NM_010824.2 |
| R: CAT AAC GGA AAG CGT TGG TG | 1698-1707 | ||
| Nlrp3 | F: AGC CTT CCA GGA TCC TCT TC | 1092-1111 | NM_145827.4 |
| R: CTT GGG CAG CAG TTT CTT TC | 1224-1243 | ||
| Nlrc4 | F: CTG GAA AAG GAT GGG AAT GA | 2815-2834 | XM_006524347.3 |
| R: CCA AGG CAG CAT CAA TGT AG | 2882-2901 | ||
| Asc | F: GAA GCT GCT GAC AGT GCA AC | 289-308 | XM_021168587.1 |
| R: GCC ACA GCT CCA GAC TCT TC | 482-501 | ||
| Gapdh | F: AAC GAC CCC TTC ATT GAC | 186-203 | NM_001289726.1 |
| R: TCC ACG ACA TAC TCA GCA C | 358-376 |
Collection and analysis of bronchoalveolar lavage fluid (BALF)
BALF was collected 1 or 2 days after the last OVA challenge and hRV infection, as described elsewhere (39). Briefly, mice were anesthetized by an intramuscular injection of Zolazepam (Zoletil 50; Virbac, Carros, France) before a thoracotomy. The trachea was revealed and punctured with scissors, followed by the insertion of a 21-gauge winged infusion set connected to a syringe containing 0.6 ml of PBS that was used to gently flush the lung. The flushing process of the lung with PBS was performed 2 more times using 1.0 ml of PBS each time. The first BALF was used for cytokine ELISA after centrifugation at 1,000 rpm for 5 min. The cells obtained from the first BALF were then combined with the cells obtained from the second and third BALFs and used for the leukocyte analysis. To analyze the differential leukocytes in the BALF, cell suspensions (1×105/100 μl) obtained from the BALF were put through Shandon Cytospinning (Thermo Scientific, Waltham, MA, USA), and then the slides were dried and stained with Wright-Giemsa stain solution (Muto Pure Chemicals Ltd., Tokyo, Japan). After that, eosinophils, neutrophils, macrophages, and lymphocytes were differentiated by cell morphology and counted using a light microscope.
ELISA for cytokines and Igs
The levels of IL-4, IL-5, IL-13, IL-25, IL-17A, IL-33, IL-1β, and IFN-γ in the BALF were determined using sandwich ELISA according to the manufacturer's protocols (eBioscience, BD Bioscience, Invitrogen). 96-well MaxiSorp plates (Nunc Immuno) were coated with 50 μl of the relevant working concentration of capture Ab in PBS overnight. The plates were washed 3 times with ELISA wash buffer (PBS containing 0.05% Tween- PBST) and then blocked with 100 μl of ELISA diluent (ELISA/ELISPOT Diluent, Invitrogen) for at least 2 hours. The BALF samples and standards for recombinant cytokine proteins were added to the plates, which were then kept overnight at 4°C. Following another washing, the relevant concentration of biotinylated Abs was added as detection Abs and incubated for 2 h at 37°C. The plates were washed with PBST, followed by incubation with streptavidin-HRP for 40 min at 37°C. Color development was then performed by adding tetramethylbenzidine. The reaction was stopped by the addition of 50 μl of stop solution (1 M phosphoric acid), and the OD values were measured at 450 nm using an ELISA reader (Molecular Devices, San Jose, CA, USA). Cytokine concentrations were calculated with SoftMax Pro5.2 using comparisons with 2 concentrations of standard cytokine proteins. The levels of OVA-specific-IgE, IgG, IgG1, and IgG2a in the sera were determined using conventional ELISA.
Measurement of airway hyperresponsiveness
Airway responsiveness was measured 24 h after the hRV-induced asthma exacerbation by recording respiratory pressure curves using barometric unrestrained whole-body plethysmography (Buxco; EMKA Technologies, Paris, France) following inhalation of methacholine (acetyl-β-methyl-choline chloride, Sigma-Aldrich) by conscious, unrestrained mice. Airway responsiveness was expressed as enhanced pause (Penh value), as described elsewhere (39). Briefly, mice were placed in a whole-body chamber, and basal readings were obtained and averaged for a 5-min period. Subsequently, increasing doses of methacholine (0, 6.25, 12.5, 25, and 50 mg/mL) were aerosolized for 1.5 min, and readings were taken and averaged for 5 min after each nebulization.
Histological examinations and periodic acid-Schiff (PAS) and trichrome staining
Histopathological examination was performed using the big lung lobe (left) perfused with 10% neutral buffered formalin (Clinic Medi Labs, Montreal, Canada). Lung tissues were embedded in paraffin 2 days after the last OVA challenge, and 10-μm sections were prepared and stained with H&E or PAS solution. Sections were analyzed by Nikon Eclipse E600 microscopy (Nikon, Tokyo, Japan). Lung inflammation and goblet cell hyperplasia were graded using a previously reported semiquantitative scoring system (40,41). To grade inflammatory cell infiltration, peri-bronchiole and peri-vascular inflammation were blind evaluated on the basis of a 5-point grading system as follows: 0, normal; 1, low grade cell influx, no tissue pathology; 2, low to moderate cell influx, low grade tissue damage; 3, moderate cell influx, low grade tissue damage; 4, moderate to high cell influx, marked tissue damage; 5, high cell influx, significant tissue pathology. Five fields were counted for each section, and the mean score from 5–6 mice per group was calculated. To quantify airway goblet cells, the following 5-point grading system was used: 0, <0.5% PAS-positive cells; 1, <25%; 2, 25%–50%; 3, 50%–75%; and 4, >75%. Five fields were counted for each section, and the mean area or mean score from 5–6 mice per group was calculated (42). Analyses were conducted in a blinded manner, and slides were presented in random order for each examination. Trichrome staining was also used to visualize and estimate collagen deposits around the airways of mice following repeated allergen and hRV exposure, as described elsewhere (43).
Flow cytometric analysis
The phenotypic analysis of infiltrated leukocytes in BALF was conducted by flow cytometry. BALF cells were washed and resuspended (0.5–1×106 cells) in 100 μl of FACS staining buffer (PBS, 2% BSA, 0.05% NaN3). Cells were stained by incubation with a properly diluted mAb cocktail at 4°C for 40 min. After staining, the cells were washed twice with FACS buffer by spinning at 1,200 rpm and 4°C for 5 min. Following fixation, cells were resuspended in PBS and analyzed using a FACS Calibur equipped with CellQuest (Becton Dickson Medical Systems, Sharon, MA, USA) and FlowJo (Tree Star, San Carlos, CA, USA) software.
Generation of bone marrow (BM) chimeric mice
BL/6 and IDO KO mice that would be used as recipients were pre-treated with busulfan (44) to deplete their BM cells. After that, the IDO KO and BL/6 recipient mice were given BM cells (1.5×107 cells/mouse) derived from BL/6 or IDO KO donor mice, respectively. The recipient mice were then given sulfamethoxazole and trimethoprim in their drinking water for the next 10 days and allowed to reconstitute their HSC population for 4 to 6 weeks. BM chimerism was confirmed prior to experimental use. To induce hRV exacerbation of acute asthmatic inflammation, the BM chimeric mice were sensitized with 100 μg of OVA in 2 mg of alum through i.p. injection. After 10 days, the BM cell recipients were challenged with either 50 μg of OVA or PBS for 3 consecutive days via the i.n. route. For hRV-induced asthma exacerbation, the third OVA challenge solution included hRV (1.0×107 TCID50). Analyses were performed 1 day after the last OVA challenge.
Allergen-specific cytokine production in lung and mediastinal lymph nodes (mLNs)
After heart perfusion to expel blood cells from the lung tissues, lung lobes were collected, chopped, and homogenized through a 100-μm mesh tissue sieve, followed by digestion with 0.5 mg/mL of collagenase type IV (Life Technologies, Grand Island, NY, USA) and 1 mg /mL of Dnase I (Amresco, Solon, OH, USA) in HBSS for 1 h at 37°C with vibration. The digested homogenates were then disrupted into a single-cell suspension by passage through a 20-gauge needle and filtering through a 45-μm nylon mesh. The harvested lung cell suspension was centrifuged at 1,500 rpm and 4°C for 5 min. The cell pellets were then re-suspended in 5.0 mL of NH4Cl-based hypotonic RBC lysis buffer for 10 min. Cells were recovered by filtration through a 70-μm nylon strainer and washed at 1,500 rpm for 7 min. The leukocytes of mLNs were harvested by gently pressing them through sterile 100-μm cell strainers. The leukocytes obtained from the lung tissues and mLNs were dispersed in a 96-well round-bottom plate (5×105 cells/well) in 100 μL of complete RPMI supplemented with 2% FBS, penicillin (100 U/mL), and streptomycin (100 U/mL). Cells were then stimulated with OVA (500 μg/mL) at 37°C for 72 h. Culture supernatants obtained from the stimulated cells were used in cytokine ELISA to evaluate CD4+ Th responses.
Analysis of CD4+ Th subsets in lung tissue and BALF
To monitor the CD4+ Th subsets in BALF and lung tissues, brief stimulation with PMA and ionomycin was used, as previously described (39). After dispensing leukocytes obtained from the lung tissues and BALF, the cells were cultured in 96-well plates (106 cells/well) with PMA (100 ng/mL) and ionomycin (1.0 μg/mL) in the presence of monensin (2 μM) at 37°C for 5 h. The stimulated cells were washed twice with PBS and surface-stained with FITC-anti-CD4 at 4°C for 30 min. After washing twice with PBS containing monensin and fixation buffer, the cells were washed twice with permeabilization buffer (eBioscience) and then stained with PE-anti-IFN-γ, PerCP-anti-IL-4, and APC-anti-IL-17A in permeabilization buffer at room temperature (RT) for 30 min. After being washed twice with PBS, the cells were fixed with fixation buffer. To monitor Treg cells, leukocytes were surface-stained with FITC-anti-CD4 markers on ice for 30 min, followed by fixation with permeabilization buffer (eBioscience) at 4°C for 6 h. After fixation, the cells were washed twice with permeabilization buffer and stained with PE-anti-Foxp3 in permeabilization buffer at RT for 30 min. The cells were then resuspended in PBS and analyzed using a FACS Calibur equipped with CellQuest (Becton Dickson Medical Systems) and FlowJo (Tree Star) software.
Statistical analysis
All data are expressed as the mean± SEM. Statistically significant differences between groups were analyzed using unpaired 2-tailed Student's t-testing for the ex vivo experiments and immune cell analyses. For multiple comparisons, statistical significance was determined using 1-way or 2-way analysis of variance with repeated measures, followed by Bonferroni post hoc testing. The statistical significance of in vivo cytokine gene expression was evaluated by unpaired 2-tailed Student's t-testing. A p-value ≤0.05 was considered significant. All data were analyzed using GraphPad Prism 4 software (GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
IDO ablation exacerbates neutrophilic airway inflammation caused by hRV infection
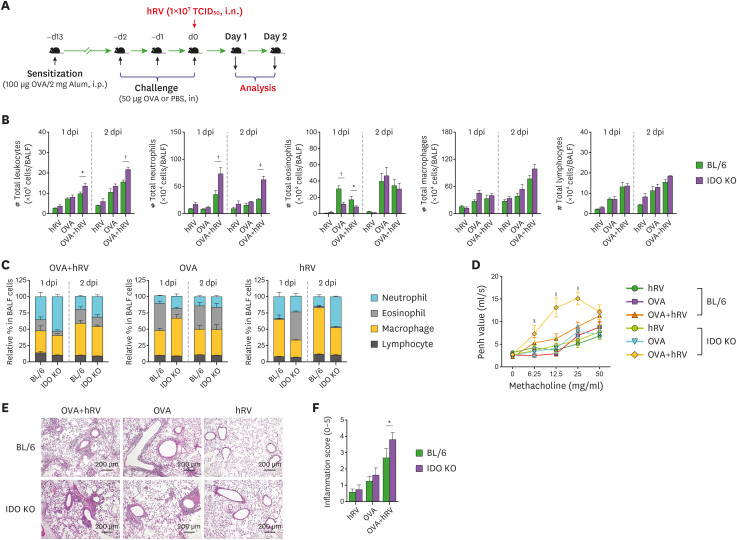
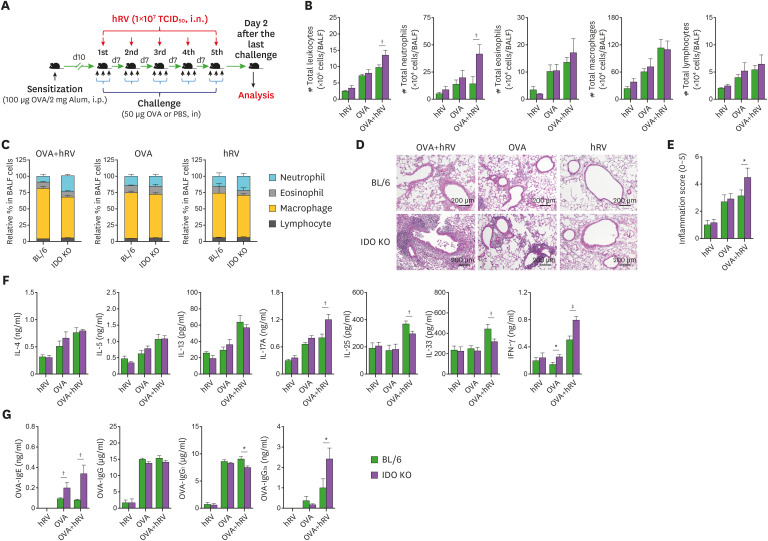
In addition to canonical Th2-mediated eosinophilic inflammation in the airway, dominant neutrophilic influx in airways inflamed by various stimuli has been also documented (3,4,5). hRV exposure is a foremost cause of steroid-resistant, neutrophilic inflammation in asthmatic patients (3,4,5,6). To investigate the role of IDO in neutrophilic inflammation during hRV-mediated asthma exacerbation, we sensitized WT BL/6 and IDO-deficient mice with OVA protein (100 μg/mouse) in 2 mg of alum via the i.p. route and then challenged those mice with 50 μg of OVA for 3 consecutive days via the i.n. route 10 days later. The asthma exacerbation, manifested as neutrophilic inflammation in the airway, was induced by infection with hRV (1×107 TCID50/mouse) through i.n. inoculation administered with the third OVA challenge. The neutrophilic airway inflammation was evaluated by analyzing the recruited leukocytes in BALF harvested 1 and 2 days after hRV infection (Fig. 1A). Our results reveal that IDO ablation resulted in an increased number of total leukocytes in the harvested BALF (Fig. 1B). Compared with the BL/6 mice, the IDO-ablated mice showed a highly increased number of neutrophils in BALF following hRV infection during the OVA challenge in our Wright-Giemsa staining analysis of the leukocyte subsets (neutrophils, macrophages, and lymphocytes). The IDO-ablated mice contained a significantly decreased number of eosinophils in BALF at 1 dpi compared with BL/6 mice, but they showed similar levels on the 2nd day. BALF macrophages and lymphocytes did not differ significantly between the BL/6 and IDO-deficient mice. In our evaluation of the relative ratio of leukocyte subsets in BALF, the infiltration of neutrophils in the IDO-deficient mice was more apparent than that in BL/6 mice after the OVA and hRV challenge (Fig. 1C). As expected, the BL/6 mice challenged with the OVA protein alone showed dominant infiltration of eosinophils in BALF, and a lack of IDO provided a modestly decreased proportion of eosinophils in BALF 1 day after the last OVA challenge. Interestingly, hRV infection without the OVA challenge appeared to induce neutrophil infiltration in IDO-deficient mice, even though the total number of neutrophils harvested from that BALF was much lower compared than that in the BL/6 and IDO mice challenged with OVA and hRV. This indicates that hRV infection induces the dominant infiltration of neutrophils in the airway. Collectively, these results indicate that IDO deficiency results in greatly increased infiltration of neutrophils in airways during asthma exacerbations caused by hRV infection.
Figure 1. Exacerbation of hRV-induced neutrophilic airway inflammation in IDO-ablated mice. (A) Schematic of asthma exacerbation by hRV infection. BL/6 and IDO KO mice were sensitized with 100 μg of OVA in 2 mg of alum through i.p. injection. After 10 days, the mice were challenged with either 50 μg of OVA or PBS for 3 consecutive days via the i.n. route. For hRV-induced asthma exacerbation, the third OVA solution included hRV (1.0×107 TCID50), and analyses were performed 1 and 2 days after the last OVA challenge. Control mice were treated with PBS. (B) The absolute number of neutrophils, eosinophils, macrophages, and lymphocytes in BALF. The leukocytes in BALF were assessed by cytospinning and subsequent Wright-Giemsa staining 1 and 2 days after the last OVA challenge. (C) Relative proportion of leukocyte subsets in BALF. (D) AHR in response to increasing doses of methacholine. Enhanced pause (Penh) values were determined by airway resistance to methacholine of 6.25, 12.5, 25, and 50 mg/ml in relation to PBS values. (E) Histological examinations of lung tissues. Representative H&E-stained lung sections derived from each group of BL/6 and IDO KO mice were examined at 2 dpi. Images are representative of sections (100×) from at least 5 mice. Represented photomicrographs show inflamed perivascular and peribronchial areas. (F) Quantitative analyses of airway inflammation in lung sections. Inflammation was blind scored 2 days after the last OVA challenge. Data show the mean±SEM of the levels derived from at least 2 individual experiments (n=5–6).
*p<0.05, †p<0.01, and ‡p<0.001 between the levels derived from BL/6 and IDO KO mice; §p<0.05 and ∥p<0.001 between the OVA-hRV challenged groups of BL/6 and IDO KO mice 1 dpi.
We measured airway responsiveness in conscious, unrestrained mice using Pehn values. As anticipated, after the administration of 12.5 and 25 mg/ml methacholine, the Pehn values increased more in the IDO-ablated mice challenged with OVA and hRV than in the identically challenged BL/6 mice (Fig. 1D). However, the IDO-ablated mice challenged with OVA alone exhibited no significant changes in Pehn values in response to methacholine inhalation compared with the OVA-challenged BL/6 mice. This implies that IDO ablation might not affect airway responsiveness after challenge with OVA alone, even though the IDO-ablated mice showed fewer eosinophils in their BALF after 1 day. To better understand severe asthmatic inflammation and disease progression in IDO-ablated mice, we performed histopathological examinations of lung tissues recovered from BL/6 and IDO KO mice at 2 dpi. As expected, the BL/6 mice showed less inflammation than the IDO KO mice, as shown by lower leukocyte infiltration in the peribronchial and perivascular areas (Fig. 1E). In particular, the IDO KO mice developed apparently severe inflammation, with huge infiltration of inflammatory cells in the airway areas. However, the BL/6 and IDO KO mice that received only the OVA challenge showed comparable inflammation in their airways. Furthermore, the IDO KO mice displayed higher inflammation scores in their airway when we scored the severity of inflammation in the airway areas (Fig. 1F). Therefore, IDO is required to regulate severe neutrophilic inflammation in airways during asthma exacerbations induced by OVA challenge combined with hRV infection.
IDO ablation facilitates Th1- and Th17-based inflammation in the airway following hRV infection
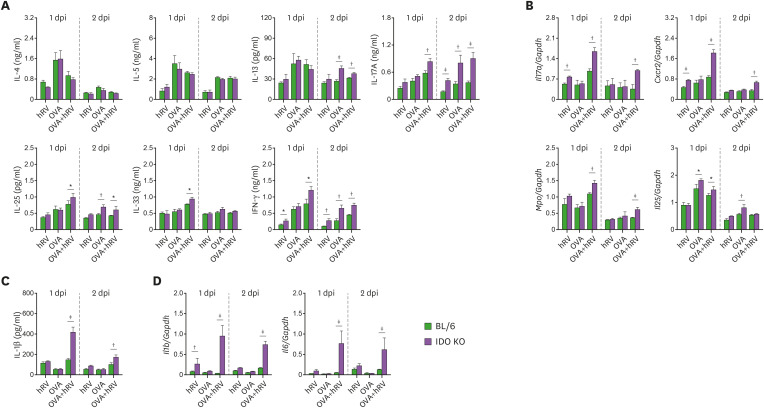
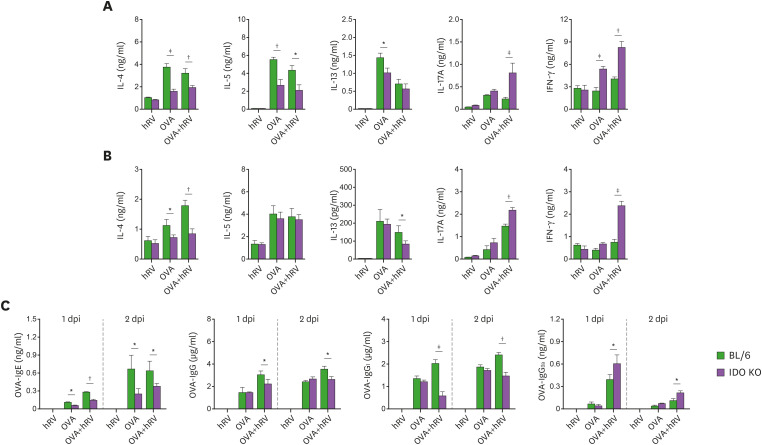
Several in vivo and ex vivo asthma models have been used to elucidate the roles of different cytokines in the differentiation and mechanistic initiation of the Th1-, Th2-, and Th17- (or even Th9) response during asthma pathogenesis (2). To characterize the Th responses in the enhanced neutrophilic airway inflammation caused by OVA and hRV infection in IDO KO mice, we determined the amount of Th subset–related cytokine proteins present in the BALF of both BL/6 and IDO-ablated mice 1 and 2 dpi. hRV infection increased the secretion of IL-13, IL-17A, IL-25, IL-33, and IFN-γ in the BALF of IDO KO mice challenged with OVA compared with BL/6 mice (Fig. 2A). In particular, IDO-ablated mice produced much higher levels of Th17- and Th1-related cytokines, such as IL-17A and IFN-γ, than BL/6 mice after the OVA challenge and hRV infection. Furthermore, IDO KO mice showed enhanced expression of Th17-inducing cytokine mRNA (IL-17A) in their lung tissues compared with the BL/6 mice (Fig. 2B). Also, enhanced expression of Cxcr2 and Mpo mRNA strengthened neutrophilic airway inflammation in IDO KO mice, along with high production of IL-17A and IFN-γ, because CXCR2 and MPO are important molecules for the functioning of neutrophils (45). Furthermore, the IDO-ablated mice had higher expression and production levels of IL-1β and IL-6, which play an important role in Th17 differentiation (Fig. 2C). These results suggest that IDO attenuates both Th17- and Th1-mediated airway inflammation during asthma exacerbations caused by hRV infection.
Figure 2. IDO-ablated mice display increased Th1- and Th17-type inflammation in the airway following hRV infection. (A) The levels of Th1, Th2, and Th17 cytokines in BALF. The protein levels of Th1 (IFN-γ), Th2 (IL-4, IL-5, IL-13, IL-25, and IL-33), and Th17 (IL-17A) cytokines were determined by cytokine ELISA using BALF collected 1 and 2 days after the last OVA challenge. (B) The expression of neutrophilic airway inflammation-related mRNA in lung tissues. The expression levels of neutrophilic airway inflammation-related mRNA (Il17a, Cxcr2, Mpo, and Il25) were determined by real-time qRT-PCR using lung tissues harvested 1 and 2 days after the last OVA challenge. (C) IL-1β protein levels in BALF. (D) The expression of Il1b and Il6 mRNA in lung tissues. IL-1β protein levels in BALF and mRNA expression of Il1b and Il6 in lung tissues were determined by ELISA and real-time qRT-PCR 1 and 2 days after the last OVA challenge, respectively. Data show the mean±SEM of the levels derived from at least 2 individual experiments (n= 5–6).
*p<0.05, †p<0.01, and ‡p<0.001 between the levels derived from BL/6 and IDO KO mice.
IDO ablation induces disrupted TJs but enhances inflammasome and mucin-inducing gene expression
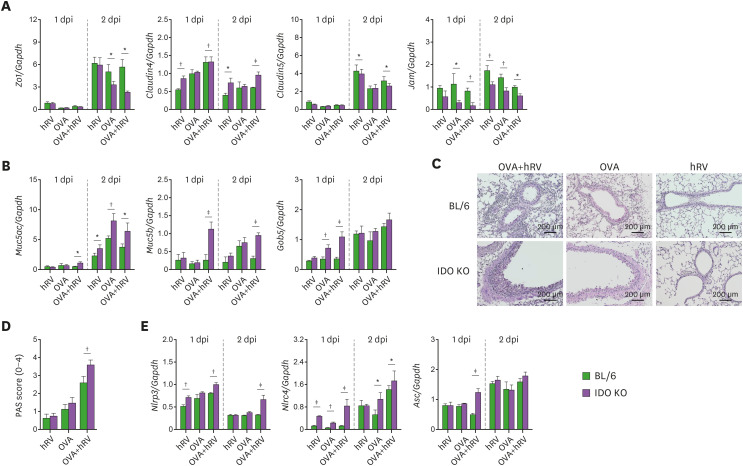
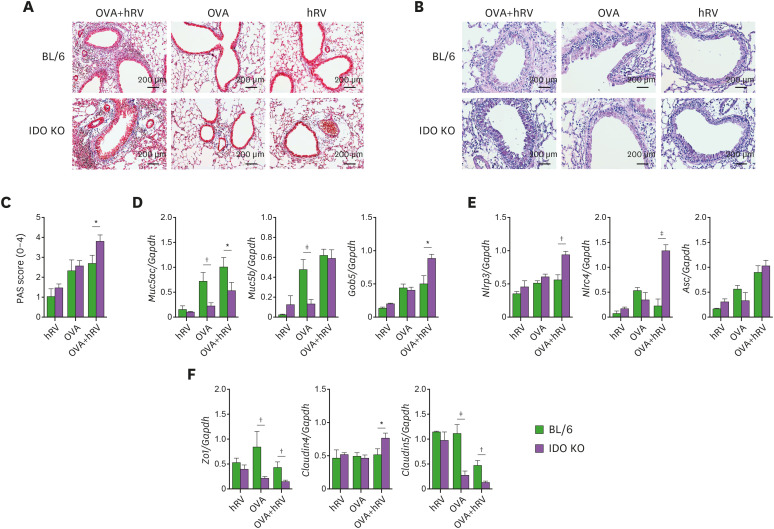
TJ proteins are responsible for airway cellular integrity and regulate the recruitment of inflammatory cells during viral infections (46). The disruption of TJs and adhesion molecules is highly linked with the infiltration of inflammatory cells upon infection with respiratory viruses (47). Furthermore, TJs such as ZO-1, claudin-4, and claudin-5 were reported to be involved in neutrophilic asthmatic reactions (47,48). Because IDO KO mice showed severe neutrophilic airway inflammation after challenge with OVA and hRV infection, we examined the mRNA expression of Zo1, claudin4, claudin5, and Jam in lung tissues. Our data reveal that IDO ablation resulted in significantly decreased expression of Zo1, claudin5, and Jam after challenge with OVA and hRV infection, but the expression of claudin-4 increased in the IDO KO mice (Fig. 3A), which supports the strong connection between severe asthma and the high expression of claudin-4 in the lungs (47). However, upon challenge with OVA alone, the BL/6 and IDO KO mice showed similar downregulated expression of Zo1, claudin4, claudin5, and Jam mRNA. This result implies that the remarkable loss of TJ integrity in IDO KO mice could be supervened by an influx of neutrophils.
Figure 3. IDO-ablated mice displayed disrupted TJs but enhanced expression of inflammasome-related and mucin-inducing genes. (A) Alteration of TJ mRNA expression. The expression of TJ mRNA (Zo1, claudin4, and claudin5) were determined by real-time qRT-PCR 1 and 2 days after the last OVA challenge. (B) Enhanced expression of Muc5ac, Muc5b, and Gob5 mRNA levels in the lung tissues of IDO KO mice. The expressions of Muc5ac, Muc5b, and Gob5 mRNA were determined by real-time qRT-PCR using lung tissues from 1 and 2 days after the last OVA challenge. (C) PAS staining of airway tissues in asthmatic mice. Lung tissue sections were obtained from BL/6 and IDO KO mice 2 days after the final OVA challenge and used for PAS staining to detect the degree of goblet cell hyperplasia and mucus plugging of the airway. (D) Scoring of the extent of mucus production. The extent of mucus production was blind scored using PAS-positive cells in the airway 2 days after the last OVA challenge. (E) The expression of inflammasome-related mRNA in lung tissues. Inflammasome-related mRNA levels were determined by real-time qRT-PCR. Data show the mean±SEM of the levels derived from at least 2 individual experiments (n=5–6).
*p<0.05, †p<0.01, and ‡p<0.001 between the levels derived from BL/6 and IDO KO mice.
Goblet cell metaplasia and subsequent mucus exaggeration is a functional signature of allergic airway inflammation (47). To further understand the role of IDO in neutrophilic airway inflammation during asthma exacerbations caused by hRV infection, we examined the expression of Muc5ac and Muc5b, which are the principal macromolecules in airway mucus (49). Highly increased levels of Muc5ac and Muc5b mRNA expression were observed in IDO-deficient mice after challenge with OVA and hRV infection (Fig. 3B). We also examined the mRNA expression of Gob5, which induces mucus protein in the airway as a member of the calcium-activated chloride channel family (47). As expected, IDO KO mice showed impressively increased expression of Gob5 mRNA in lung tissues 1 day after challenge with OVA and hRV infection (Fig. 3B). Those data were supported by the PAS staining results, wherein the lung tissues of IDO KO mice had more mucus production (Fig. 3C) and higher PAS scores than those of BL/6 mice (Fig. 3D). Collectively, our data indicate that IDO ablation promotes severe neutrophilic airway inflammation with abundant production of mucus during asthma exacerbations. Severe neutrophilic asthma is highly connected to inflammasome activation and concomitant IL-17 and IL-1β induction in inflamed airways (50). Hindering NLRP3 inflammasome activation was reported to reduce neutrophilic asthma but have no effect on the eosinophil-mediated Th2 response (50). Similarly, our data reveal that the IDO KO mice showed higher expression of Nlrp3, Nlrc4, and Asc mRNA in their lung tissues than BL/6 mice after challenge with OVA and hRV infection (Fig. 3E). Taken together, these results support that the neutrophilic asthmatic features of OVA-challenged and hRV-infected IDO KO mice occur through the intense induction of inflammasome activation and mucus secretory molecules and the collapse of the lung epithelial barrier.
IDO deficiency enhances allergen-specific Th1- and Th17-biased CD4+ T cell responses following hRV infection
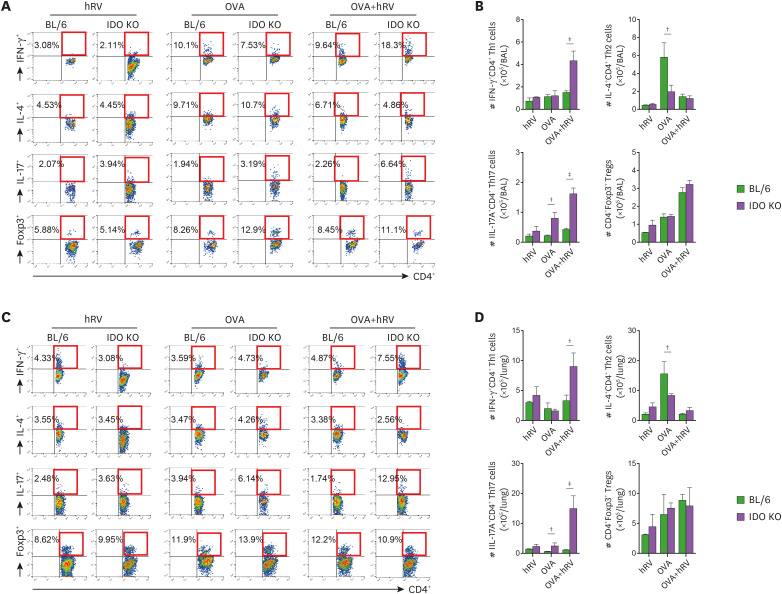
To clearly understand the skewed Th17 and Th1 responses in IDO KO mice, we further examined the CD4+ Th subsets that infiltrated their inflamed BALF and lung tissues 1 dpi. Compared with that of the BL/6 mice, the BALF of the IDO KO mice contained a higher frequency and absolute number of IL-17A+CD4+ Th17 and IFN-γ+CD4+ Th1 cells after challenge with OVA and hRV infection (Fig. 4A and B). However, IL-4+CD4+ Th2 cells were detected with a lower frequency and absolute number in BALF, and especially in IDO KO mice, after challenge with OVA alone. Thus, IDO could play a role in regulating the differentiation of effector IL-4+CD4+ Th2 cells. CD4+Foxp3+ Treg cells did not change significantly, even though CD4+Foxp3+ Treg cells were detected at slightly increased levels in the IDO KO mice. Similarly, after challenge with OVA alone, the lung tissue of IDO KO mice contained a higher frequency and absolute number of IL-17A+CD4+ Th17 and IFN-γ+CD4+ Th1 cells than that of BL/6 mice, whereas the levels of IL-4+CD4+ Th2 cells in IDO KO mice decreased (Fig. 4C and D). These results indicate that IDO ablation could provide a skewed response of IL-17- and IFN-γ-producing CD4+ T cells in inflamed tissues.
Figure 4. IDO deficiency derives enhanced Th1- and Th17-biased CD4+ T cell responses at inflamed sites. (A) and (B) The frequency and number of CD4+ Th subsets in BALF. BALF was collected 1 day after the last OVA challenge. (C) and (D) The frequency and number of CD4+ Th subsets in lung tissues. Lung leukocytes were recovered by heart perfusion and collagenase digestion 1 day after the last OVA challenge. The frequency and enumeration of the CD4+ Th subsets in BALF and lung tissues were determined by intracellular cytokine staining combined with surface CD4 staining after brief stimulation of the prepared BALF and lung leukocytes with PMA and ionomycin. CD4+FoxP3+ Tregs were determined by a flow cytometric analysis using Foxp3-specific Ab without stimulation. Values in the dot-plots represent the average percentage of each population after gating on CD4+ T cells. Data show the mean±SEM of the levels derived from at least 2 individual experiments (n=4–6).
*p<0.01, †p<0.001, between the levels derived from BL/6 and IDO KO mice.
CD4+ Th2 primed with OVA absorbed to alum are activated in draining LNs (mLNs), which then migrate to the lung to coordinate the asthmatic immune response during an OVA challenge (51). However, our results reveal that skewed Th17 and Th1 responses were dominant in the airways of the IDO KO mice after an OVA challenge and hRV infection. Therefore, whether the skewed Th17 and Th1 responses in IDO KO mice are induced in mLNs following the OVA plus hRV challenge or by Th17- and Th1-biased migration in the inflamed tissues is unclear. To further understand OVA-specific CD4+ Th responses, we examined the production of Th subset-related cytokines in leukocytes obtained from mLNs and lung tissues following stimulation with the OVA protein, which could induce dominant cytokine production from CD4+ T cells. In line with the cytokine levels in the BALF, the IDO KO mice challenged with OVA and hRV infection showed markedly increased production of Th17- and Th1-related cytokines (IL-17A and IFN-γ) from CD4+ T cells derived from mLNs and lung tissues in response to OVA stimulation, but the production of the Th2-related cytokines, IL-4, IL-5, and IL-13, decreased (Fig. 5A and B). The strong and complex relationships among the allergen-specific IgE, IgG, IgG1, and IgG2a responses with Th in the progression of allergic inflammation have been documented (52). Therefore, we measured the levels of OVA-specific IgE, IgG, IgG1, and IgG2a in sera. In support, the IDO KO mice showed lower levels of OVA-specific IgE, IgG, and IgG1 and modestly increased levels of OVA-specific IgG2a compared with the BL/6 mice (Fig. 5C). Therefore, the skewed Th17 and Th1 responses observed in the IDO-ablated mice could be driven by biased activation of Th17 and Th1 cells in mLNs and inflamed lung tissue.
Figure 5. Enhanced responses of allergen-specific CD4+ Th1 and Th17 cells in IDO-ablated mice. (A) and (B) OVA-specific production of Th1, Th2, and Th17 cytokines from mLNs and lung leukocytes. The leukocytes were prepared from mLNs (A) and lung tissues (B) using collagenase digestion 1 day after the last OVA challenge and stimulation with OVA (500 μg/mL) for 72 h. The protein levels of Th1 (IFN-γ), Th2 (IL-4, IL-5, IL-13), and Th17 (IL-17) were then determined by cytokine ELISA using culture soup. (C) Serum levels of IgE, IgG, IgG1, and IgG2a. OVA-specific Ig levels in sera were measured by sandwich ELISA 1 and 2 days after the final OVA challenge. Data show the mean±SEM of the levels derived from at least 2 individual experiments (n=4–6).
*p<0.05, †p<0.01, and ‡p<0.001, between the levels derived from BL/6 and IDO KO mice.
IDO ablation provokes marked exacerbation of Th17- and Th1-based airway inflammation with repeated allergen and hRV exposure
Long-term recurrent stimulation with an allergen can produce chronic airway inflammatory responses that manifest as the destruction of the epithelial structure and eventually asthma airway remodeling (53,54). The mechanisms of allergen-specific Th2-mediated and chronic asthma pathogenesis are somewhat understood, but chronic exacerbation of asthmatic inflammation with hRV infection remains to be explained. Therefore, to better understand the role of IDO in asthma exacerbation caused by hRV infection, we used long-term recurrent stimulation with OVA to investigate how IDO ablation affects the chronic exacerbation of asthmatic inflammation caused by hRV infection. We sensitized BL/6 and IDO-ablated mice with 100 μg of OVA in 2 mg of alum via the i.p. route and then challenged them with 50 μg of OVA for 3 consecutive days per week via the i.n. route for 5 weeks. The chronic exacerbation of asthmatic inflammation was induced by infection with hRV (1×107 TCID50) during the third OVA solution each week. The features of asthmatic inflammation in the airway were evaluated by analyzing the recruited leukocytes found in BALF 2 days after the last OVA challenge with hRV infection (Fig. 6A). Our results reveal that IDO ablation resulted in a somewhat increased number of total leukocytes in the harvested BALF (Fig. 6B). In addition, the IDO-ablated mice showed a highly increased number of neutrophils in BALF following repeated OVA challenge and hRV infection, but the number of other leukocyte subsets (eosinophils, macrophages, and lymphocytes) did not change significantly compared with the BL/6 mice. Compared with the BL/6 mice, the IDO-ablated mice showed a pattern of increased neutrophils in the relative ratio of total leukocytes derived from BALF following repeated OVA challenge and hRV infection, even though the BALF of both the BL/6 and IDO KO mice contained a dominant population of macrophages (Fig. 6C). The relative ratio of eosinophils was found to be comparable in the BL/6 and IDO KO mice, and it is noteworthy that challenging IDO KO mice with hRV alone appeared to increase the infiltration of neutrophils. These results indicate that IDO ablation results in predominantly increased infiltration of neutrophils in airways during the chronic exacerbation of asthmatic inflammation caused by repeated OVA challenge and hRV infection. To better understand the chronic exacerbation of asthmatic inflammation in IDO KO mice, we performed histopathological examinations of lung tissues recovered from BL/6 and IDO KO mice after repeated OVA challenges and hRV infections. As expected, the IDO-ablated mice showed more exacerbated inflammatory lesions around the airway areas than the BL/6 mice (Fig. 6D). Notably, IDO-ablated mice showed hyperplasia of the epithelial layers in the peribronchial and perivascular areas after repeated OVA challenges and hRV infections. In support, the IDO KO mice displayed higher inflammation scores in their airways (Fig. 6E). However, no significant changes in inflammation score were found in BL/6 and IDO KO mice following repeated challenges with OVA alone. Thus, IDO might not be involved in regulating the chronic asthmatic inflammation caused by repeated challenge with Th2-mediated allergens. Next, we determined the secretion levels of Th-related cytokines in BALF derived from BL/6 and IDO KO mice following repeated OVA challenges and hRV infections. In line with the enhanced infiltration of neutrophils that we found in the IDO KO mice, the IDO KO mice showed highly increased secretion of Th17- and Th1-related cytokines (IL-17A and IFN-γ) in BALF compared with the BL/6 mice, but the Th2-related cytokines (IL-4, IL-5, and IL-13) were detected at comparable levels in the BALF derived from the BL/6 and IDO KO mice (Fig. 6F). Interestingly, the IDO KO mice had decreased secretion of IL-25 and IL-33, which are produced by epithelial cells and known to be involved in innate lymphoid cell 2 differentiation, compared with BL/6 mice. We measured the levels of OVA-specific IgE, IgG, IgG1, and IgG2a to further understand the features of chronic asthmatic inflammation caused by the repeated OVA challenges and hRV infections. We found no changes in the levels of OVA-specific IgG and IgG1, but the IDO KO mice showed higher levels of OVA-specific IgG2a (Fig. 6G). Also, it was curious that IDO KO mice contained higher levels of OVA-specific IgE than BL/6 mice. Collectively, these results indicate that IDO ablation exacerbates the features of chronic asthmatic inflammation mediated by Th17- and Th1-type responses following repeated OVA challenges and hRV infections.
Figure 6. IDO-ablated mice show exacerbated Th1- and Th17-type airway inflammation following repeated allergen and hRV exposure. (A) Experimental scheme for chronic model of hRV-induced asthma exacerbation. BL/6 and IDO KO mice were sensitized twice with 50 μg of OVA in 2 mg of alum on days 0 and 7 through i.p. injection. Ten days later, the mice were challenged with either 50 μg of OVA or PBS for 3 consecutive days via the i.n. route. For hRV-induced asthma exacerbation, the third OVA solution included hRV (1.0 × 107 TCID50), and that 3-consecutive-days challenge was repeated 5 times every 7 days. The analysis of leukocyte infiltration and inflammatory reactions in lung tissues was conducted 2 days after the final OVA challenge. (B) Differential cell numbers in BALF recovered from BL/6 and IDO KO mice in the chronic model of hRV-induced asthma exacerbation. The leukocyte subsets (neutrophils, eosinophils, macrophages, and lymphocytes) in BALF were assessed by cytospinning and subsequent Wright-Giemsa staining 2 days after the final OVA challenge. (C) Relative proportion of leukocyte subsets in BALF. (D) Histological examinations of lung tissues. Representative H&E-stained lung sections derived from each group of BL/6 and IDO KO mice 2 days after the final OVA challenge were examined. Images are representative of sections (100×) from at least 5 mice. The photomicrographs show inflamed perivascular and peribronchial areas. (E) Quantitative analyses of airway inflammation in lung sections. Inflammation was blind scored 2 days after the last OVA challenge. (F) The levels of Th1, Th2, and Th17 cytokines in BALF. The protein levels of Th1 (IFN-γ), Th2 (IL-4, IL-5, IL-13, IL-25, and IL-33) and Th17 (IL-17A) cytokines were determined by cytokine ELISA using BALF collected 2 days after the last OVA challenge. (G) Serum levels of IgE, IgG, IgG1, and IgG2a. OVA-specific Ig levels in sera were measured by sandwich ELISA 2 days after the final OVA challenge. Data show the mean±SEM of the levels derived from at least 2 individual experiments (n= 4–6).
*p<0.05, †p<0.01, and ‡p<0.001, between the levels derived from BL/6 and IDO KO mice.
IDO deficiency results in increased sub-epithelial collagen deposition following repeated allergen and hRV exposure
The long-term recurrent exposure of IDO KO mice to allergens and hRV exacerbated chronic inflammatory disease mediated by Th17- and Th1-type responses in the airway compared with WT mice. One of the hallmarks of chronic airway inflammation following repeated allergen exposure is structural changes in the airways, including collagen deposition, goblet cell hyperplasia, and increased smooth muscle mass (53). To better understand the characteristics of chronic airway inflammation in IDO KO mice following repeated allergen and hRV exposure, we evaluated collagen deposits in the airways with Masson's trichrome staining of lung tissues. The IDO KO mice showed denser and thicker collagen layers around the airway than the WT mice (Fig. 7A). Also, the IDO KO mice exhibited more apparent PAS+ goblet cells in the airways (Fig. 7B) and higher PAS scores than the WT mice (Fig. 7C). In contrast, the mucus production and PAS scores derived from WT and IDO KO mice did not differ significantly following repeated exposure to OVA alone. We also examined the expression of Muc5ac, Muc5b, and Gob5 mRNA in lung tissues to understand the chronic inflammatory response in the airways following repeated OVA and hRV exposure. We found increased expression of Gob5 mRNA, but not Muc5ac or Muc5b, in IDO KO mice compared with WT mice (Fig. 7D). This implies that IDO ablation could affect the expression of Gob5 during the chronic inflammation caused by repeated exposure to OVA and hRV infection, leading to goblet cell hyperplasia. Our data also reveal that IDO KO mice had enhanced expression of Nlrp3 and Nlrc4 mRNA in lung tissues compared with WT mice (Fig. 7E). That result strengthens our finding of neutrophilic airway inflammation in IDO KO mice following repeated allergen and hRV exposure because those inflammasome-activated genes are well connected to neutrophilic asthmatic features in inflamed airways (50). The IDO KO mice also showed enhanced expression of TJs related to neutrophilic airway inflammation such as Zo1, claudin5, and claudin4 compared with WT mice (Fig. 7F). Taken together, these results indicate that IDO ablation worsens neutrophilic airway inflammation, collagen deposition, and goblet cell hyperplasia and enhances the expression of inflammasome-related genes during the chronic airway inflammation caused by repeated exposure to allergens and hRV infection.
Figure 7. IDO-ablated mice show increased sub-epithelial collagen deposition and mucin-inducing gene expression. (A) Trichrome staining for type I collagen deposition in the lung tissues of BL/6 and IDO KO mice following repeated allergen and hRV exposure. (B) PAS staining of airway tissues in BL/6 and IDO KO mice following repeated allergen and hRV exposure. Photomicrographs of Masson trichrome and PAS-stained lung sections derived from each group of BL/6 and IDO KO mice were obtained 2 days after the final OVA challenge. Images are representative of sections (100×) from at least 5 mice. (C) Scoring of the extent of mucus production. The extent of mucus production was blind scored using PAS-positive cells in the airway 2 days after the last OVA challenge. (D) Altered expression of Muc5ac, Muc5b, and Gob5 mRNA levels in the lung tissues of IDO KO mice. The expressions of Muc5ac, Muc5b, and Gob5 mRNA were determined by real-time qRT-PCR using lung tissues from 2 days after the last OVA challenge. (E) The expression of inflammasome-related mRNA in lung tissues. (F) Alteration of TJ mRNA expression. The expressions of inflammasome-related and TJ mRNA were determined by real-time qRT-PCR using total RNA extracted from lung tissues sampled 2 days after the last OVA challenge. Data show the mean±SEM of the levels derived from at least 2 individual experiments (n=4–6).
*p<0.05, †p<0.01, and ‡p<0.001, between the levels derived from BL/6 and IDO KO mice.
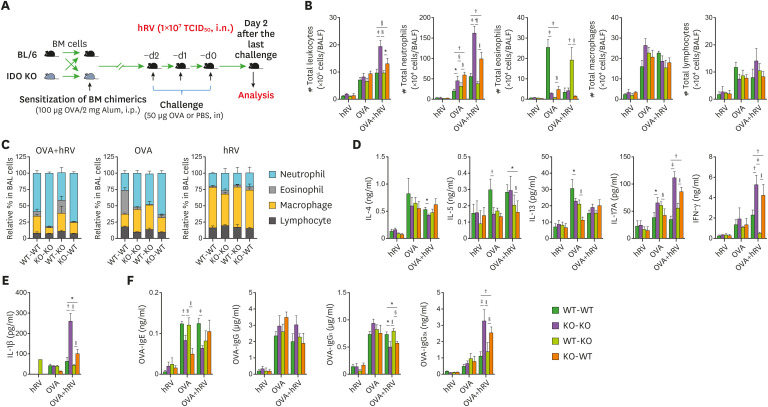
IDO from leukocytes derived from the HSC lineage plays a major role in suppressing Th1- and Th17-type airway inflammation after hRV infection
IDO is widely expressed in human tissues and various cell subsets, and it is induced at sites of inflammation and by immune cells activated by type I (IFN-I) and II IFN (IFN-II) because mammalian IDO genes contain IFN response elements (23). Elevated IDO expression by DCs is of particular significance because DCs play a crucial role in initiating Ag-specific responses (55,56). Our data reveal that IDO ablation markedly exacerbates neutrophilic airway inflammation during acute and chronic allergen exposure with hRV infection. In this analysis, we were interested in dissecting the contributions that IDO induced in tissue-resident stromal and epithelial cells and HSC-derived leukocytes make to this process. Therefore, we used a BM chimeric model of WT BL/6 and IDO KO mice. Following the depletion of BM cells in the recipients with an injection of busulfan (44), BM cells derived from BL/6 and IDO KO mice were grafted into IDO KO and BL/6 recipient mice, respectively. Five to 6 weeks later, after confirming BM chimerism, we sensitized the recipients with OVA protein (100 μg/recipient) in 2 mg of alum via the i.p. route. Ten days after that, we challenged those mice with 50 μg of OVA for 3 consecutive days via the i.n. route. Neutrophilic asthma exacerbation was induced by hRV infection with the third OVA treatment. Neutrophilic airway inflammation was evaluated by analyzing the recruited leukocytes in BALF harvested 2 days after the hRV infection (Fig. 8A). Interestingly, our data reveal that the WT recipients of the IDO KO BM donor cells (KO-WT chimera) showed an increased number of total leukocytes in BALF harvested after OVA challenge with hRV infection, compared with the IDO KO recipients of WT BM donor cells (WT-KO chimera) (Fig. 8B). In particular, the KO-WT chimera exhibited a highly increased number of neutrophils in BALF compared with the WT-KO chimera. This result indicates that IDO production in leukocytes derived from the HSC lineage plays a more important role than that from tissue-resident cells in regulating neutrophilic airway inflammation. It was also interesting to note that WT-KO recipients contained a highly increased number of eosinophils in their BALF after exposure to OVA and hRV, compared with the WT-WT, KO-WT, and KO-KO chimera. These data imply that IDO production from tissue-resident cells might play a role in recruiting eosinophils into inflamed tissues. The infiltration of neutrophils in the BALF of the KO-WT chimera was particularly apparent in our evaluation of the relative ratio of leukocyte subsets (Fig. 8C). Notably, the KO-WT and KO-KO chimera showed comparable proportions of neutrophils in their BALF, which indicates that the ablation of IDO production in leukocytes derived from HSCs plays a dominant role in inducing neutrophilic airway inflammation. To better understand neutrophilic airway inflammation in the KO-WT chimera, we determined the levels of Th1-, Th2-, and Th17-type cytokines in BALF. As expected, the KO-WT and KO-KO chimeras showed higher levels of IL-17A and IFN-γ than the WT-WT and WT-KO chimeras after exposure to OVA and hRV infection, whereas all the BM chimeras had comparable levels of Th2-type cytokines (IL-4, IL-5, and IL-13) (Fig. 8D). It is also noteworthy that IFN-γ was detected in the BALF of the WT-KO chimera at greatly reduced levels after the OVA challenge and hRV infection compared with the other BM chimeras, which seems likely to contribute to the infiltration of eosinophils in the BALF of the WT-KO chimera. Furthermore, our data show that the IL-1β in the BALF of KO-WT chimera was detected with higher levels than those in the WT-KO chimera (Fig. 8E), which supports that Th17-type airway inflammation is dominantly mediated by IDO produced from leukocytes derived from HSCs. Finally, we examined OVA-specific IgE, IgG, IgG1, and IgG2a because a previous study described a strong relationship between allergen-specific Ig isotypes and Th responses during the progression of allergic inflammation (52). In support of that report, our data reveal that the KO-WT chimera showed higher levels of OVA-specific IgG2a than the WT-KO chimera, whereas the levels of OVA-specific IgG1 were decreased in the KO-WT chimera compared with the WT-KO chimera (Fig. 8F). Collectively, these results indicate that IDO ablation in leukocytes derived from HSCs induces neutrophilic airway inflammation during asthma exacerbation caused by hRV infection.
Figure 8. IDO deficiency in hematopoietic stem cells enhances Th1- and Th17-type airway inflammation following allergen and hRV exposure. (A) Experimental scheme for hRV-induced asthma exacerbation using BL/6 and IDO KO BM chimeric models. BM cells derived from BL/6 and IDO KO mice were grafted into IDO KO and BL/6 mice, respectively, pre-treated with busulfan to deplete BM cells in the recipients. After confirmation of BM chimerism, the recipients were sensitized with 100 μg of OVA in 2 mg of alum through i.p. injection. After 10 days, the recipients were challenged with either 50 μg of OVA or PBS for 3 consecutive days via the i.n. route. For hRV-induced asthma exacerbation, the third OVA solution included hRV (1.0×107 TCID50), and analyses were performed 1 day after the last OVA challenge. Control mice were treated with PBS. (B) Differential cell numbers in BALF recovered from the recipients of BL/6 or IDO KO BM cells. The leukocyte subsets (neutrophils, eosinophils, macrophages, and lymphocytes) in BALF were assessed by cytospinning and subsequent Wright-Giemsa staining 1 day after the final OVA challenge. (C) Relative proportion of leukocyte subsets in BALF derived from BM cell recipients. (D) The levels of Th1, Th2, and Th17 cytokines in BALF. (E) IL-1β levels in the BALF of the recipients. The protein levels of the cytokines were determined by cytokine ELISA using BALF collected from the recipients of BL/6 or IDO KO BM cells 1 day after the last OVA challenge. (F) OVA-specific IgE, IgG, IgG1, and IgG2a levels in sera from the recipients. OVA-specific Ig levels in sera were measured by sandwich ELISA 1 day after the final OVA challenge. Data show the mean±SEM of the levels derived from at least 2 individual experiments (n= 4–6).
*,§p<0.05, †,∥p<0.01, and ‡,¶p<0.001, between the indicated groups.
DISCUSSION
As the common cold virus, hRV is the most prevalent pathogen to circulate constantly in the human community, and it is known to be the most important trigger of asthma exacerbations (3,4,5,6). Even though murine models infected with minor or major groups of hRV have been used to depict several mechanisms that could explain asthma exacerbations (39,57,58), a better understanding of personal and environmental risk factors and inflammatory mechanisms is needed to develop new strategies that can prevent and treat asthma exacerbations. In this study, we have demonstrated that IDO is required to prevent neutrophilic airway inflammation during asthma exacerbations caused by hRV infection. Using an OVA-based asthma model, we have shown that IDO-deficient mice had markedly enhanced Th17- and Th1-type neutrophilia in the airway following OVA challenge and hRV infection. This neutrophilic airway inflammation was closely associated with disrupted TJs and enhanced expression of inflammasome-related molecules and mucin-inducing genes. In addition, IDO ablation enhanced allergen-specific Th17- and Th1-biased CD4+ T-cell responses upon hRV infection. The role of IDO in attenuating Th17- and Th1-type neutrophilic airway inflammation became more apparent in chronic asthma exacerbations caused by repeated allergen challenges and hRV infections. IDO-deficient mice showed severely exacerbated Th17- and Th1-type neutrophilic inflammation in their airways following repeated OVA challenges and hRV infections, as manifested by marked neutrophil infiltration, high production of IL-17A and IFN-γ, and enhanced collagen deposition and epithelial hyperplasia. Furthermore, IDO in leukocytes derived from the HSC lineage appeared to play a dominant role in attenuating Th17- and Th1-type neutrophilic inflammation in the airway following hRV infection. Therefore, IDO in HSC-derived leukocytes is required to regulate Th17- and Th1-type neutrophilic inflammation in the airway during asthma exacerbations caused by hRV infections.
IDO, an IFN-γ-inducible enzyme, has traditionally been recognized for its regulatory role in infection, autoimmunity, cancer, pregnancy, and transplantation. The tolerance functions of IDO are based on the depletion of TRP and the accumulation of its metabolites KYN and 3-hydroxyanthranilic acid (3-HAA) in the intracellular pool or microenvironment of immune cells (59). However, the effect of IDO on allergic inflammation appears to be a piece of the puzzle in the “hygiene hypothesis.” The currently accumulated data provide complicated results from which no clear conclusion can be drawn. IDO-ablated mice were shown to experience significant relief from the establishment of allergic airway disease with no impairment of airway tolerance (31), which suggests that IDO plays a role in promoting Th2-mediated allergic airway inflammation. In contrast, Dr. Raz's group demonstrated that IDO-inducing molecules such as the TLR9 ligand can inhibit the airway inflammation driven by Th2 cells in the lung (32). IDO activity expressed by resident lung cells, rather than by pulmonary DCs, was suggested to suppress lung inflammation and airway hyperreactivity. Also, that group showed that 3-HAA, a metabolite of TRP catabolized by IDO, suppressed experimental asthma by inducing T cell apoptosis through the inhibition of PDK1 activation (60). Our results favor the former study results, because IDO-ablated mice challenged with OVA alone showed a reduced number of infiltrating eosinophils and reduced Th2-type cytokine production from leukocytes derived from LNs and lung tissue in response to allergen stimulation. The IDO-ablated mice in this study exhibited no significant changes in airway responsiveness following challenge with OVA alone, which means that IDO could play no role in attenuating airway responsiveness after OVA challenge alone. The newest finding in our results explores the role of IDO in developing OVA-based asthma exacerbations caused by hRV infection. Our data clearly show that IDO deficiency provides markedly enhanced Th17- and Th1-type airway inflammation in the course of hRV-induced asthma exacerbation. It has been reported that infections with respiratory viruses such as RSV and influenza induce IDO expression (36,37), but no previous study had used practical models infected by a major cause of asthma exacerbation to examine the role of IDO in asthma exacerbation. The IDO-ablated mice displayed Th17- and Th1-type airway inflammation, as manifested by large increases in neutrophil infiltration, enhanced airway responsiveness, and increased production of Th17- and Th1-type cytokines. In addition, our data reveal that leukocytes derived from lung-draining LNs and lung tissues produced Th17- and Th1-type cytokines at increased levels in response to allergen stimulation. These data could be strengthened by previous results from experimental hRV infections showing that baseline pulmonary IDO activity was lower in patients with allergic asthma than in healthy individuals (38). In line with a previous report (31), our IDO-ablated mice showed no impairment in airway tolerance, but they displayed reduced Th2-type responses in lung draining LNs and lung tissues in response to allergen stimulation. In contrast with their Th2-type response, our IDO-ablated mice displayed highly enhanced allergen-specific Th17- and Th1-type responses. Even though our data provide no mechanistic clue about how to mount Th17- and Th1-skewed responses in LNs and lung tissues, the Th17- and Th1-skewed responses we found could contribute to neutrophilic airway inflammation during an asthma exacerbation following hRV infection. The metabolites of TRP, such as KYN, 3-hydroxykynurenine, and xanthurenic acid, could affect the differentiation of CD4+Foxp3+ Treg cells during allergic inflammation (61). CD4+Foxp3+ Treg and IL-17A+CD4+ Th17 cells are derived from the same precursor cells (62), and increasing the action of IDO is considered to switch the differentiation of those precursor T cells into CD4+Foxp3+ Treg cells rather than IL-17+CD4+ Th17 cells (63,64). Therefore, the altered catabolism of TRP in IDO deficiency might affect the differentiation and balance of IL-17A+CD4+ Th17 and CD4+Foxp3+ Treg cells. Furthermore, a significant correlation was reported between IDO activity and Th17/Treg imbalance in children with allergic asthma (35). In our results, however, we did not observe a significant change in the frequency or number of CD4+Foxp3+ Treg cells in inflamed lung tissue or BALF derived from WT and IDO-deficient mice after OVA challenge and hRV infection. CD4+Foxp3+ Treg cells do not appear to be involved in accelerating the neutrophilic airway inflammation caused by hRV infection on the background of IDO deficiency. Explicating the role of CD4+Foxp3+ Treg cells during an asthma exacerbation with IDO deficiency and an in-depth study of the role of TRP metabolites in regulating the differentiation and balance of Th17 and Treg cells remain for future researchers.
Another intriguing finding in this study is that IDO activity in HSC-derived leukocytes plays a dominant role in attenuating neutrophilic airway inflammation in the course of asthma exacerbations following hRV infection. IDO can be induced in various immune cells, including DCs; other APCs; macrophages stimulated by soluble cytokines such as IFN-γ, type I IFNs, TGF-β, and TNF-α; or TLR ligands such as LPS and CpG (21,22,23,24,25). IDO induced by the TLR9 ligand was reported to suppress experimental asthma, and IDO expressed by resident lung cells rather than pulmonary DCs played a role in suppressing lung inflammation and airway hyperactivity (32). IDO enzymatic induction in resident lung cells depended on IFN-γ and IL-12p40 following stimulation with the TLR9 ligand (32). That previous report differs from our conclusion that IDO activity in HSC-derived leukocytes is important to attenuating Th17- and Th1-type neutrophilic airway inflammation during asthma exacerbations caused by hRV infections. Conceivably, this discrepancy could derive from the context of the experimental asthma model. In this study, we used BM chimeric models to dissect the contribution of IDO induced in resident lung cells from that induced in HSC-derived leukocytes during an asthma exacerbation following hRV infection. Indeed, elevated IDO expression by DCs is of particular significance due to their Ag-presenting functions. Therefore, a lack of IDO expression in DCs derived from HSCs could affect the nature of CD4+ Th cells specific for the allergen. In support, our IDO-ablated mice showed markedly enhanced CD4+ Th17 and Th1 responses in lung-draining LNs following stimulation with OVA, which suggests that IDO ablation could induce Th17- and Th1-skewed responses during allergen sensitization. The enhancement of Th17 and Th1 responses mediated by IDO-deficient DCs derived from HSCs is presumed to be involved in neutrophilic airway inflammation in the course of asthma exacerbations. Second, the lack of IDO expression in neutrophils could change their migration and apoptosis (65,66,67), thereby enhancing the infiltration of neutrophils in the airways of the WT recipients that received BM donor cells derived from IDO KO mice. That notion is supported by the result that pharmacologic inhibition of IDO increases the transepithelial migration of neutrophils (66). We deem IDO expression in HSC-derived leukocytes to be important in developing future strategies for treating asthma exacerbations.
The elevated number of eosinophils in nasal lavage fluid and BALF is related to allergic airway inflammation and respiratory viral infections that can lead to acute or chronic asthma (68,69). Highly increased neutrophil infiltration in the airway was observed in chronic asthma exacerbations driven by repeated allergen challenges and hRV infections, as was increased expression of IL-17A, IL-1β, and IFN-γ and enhanced inflammasome-activated gene expression and TJ disruption. These results suggest a mechanistic link between an increase in the inflammasome-Th17 axis and impaired barrier function. IL-1β can induce the development of IL-17-producing CD4+ Th17 cells, in cooperation with IL-23 (70). In agreement with our observations, IL-17A was found to stimulate mucin genes, Muc5ac, Muc5b, and Gob-5, in lung tissues. In addition, the inflammasome is also a potential regulator of goblet cell secretion (71). Together, these data suggest that complex barrier dysfunction could be the consequence of inflammasome activation and IL-1β/IL-17-mediated neutrophil infiltration in neutrophilic asthma. Consistently, IL-17A is known to significantly help in the maturation, migration, and function of neutrophils through enhanced granulopoiesis (72), whereas impaired IL-17A signaling is related to a delayed and insufficient neutrophil accumulation in asthmatic lungs (73). In addition, severe neutrophilic asthma is characterized by the profuse induction of mucin-producing Muc5b and Gob-5 and the concurrent loss of TJ and adhesion-molecule integrity (74). Similarly, we found that neutrophilic asthma exacerbated by hRV infection in IDO-ablated mice resulted in abundant Muc5b and Gob-5 production. Moreover, diminished Zo1, claudin4, claudin5, and Jam expression correlated with exacerbated asthmatic features in both the acute and chronic asthma models in IDO-ablated mice. Chronic asthmatic inflammation is characterized by an influx of inflammatory cells, the overproduction of mucus, and progressive fibrosis of inflamed lungs (53,54). In line with those notions, intense airway inflammation, mucus production, goblet cell disintegration, and subsequent collagen deposition were observed in our IDO-ablated mice following repeated allergen and hRV exposure. One curious thing was that IDO correlated positively with a huge infiltration of eosinophils, along with the induction of OVA-specific IgE, IgG, and IgG1 in sera sampled during acute asthma exacerbation, but an intense induction of OVA-specific IgE was observed in IDO-ablated mice following repeated allergen and hRV exposure. Asthma pathogenesis and prolonged asthmatic responses are highly associated with the activation of FcεRI-mediated mast cells and basophils to accelerate IgE isotype switching by presenting allergens among the membrane receptors of DCs (52). Class switching of IgE production appears to critically potentiate asthma pathogenesis and lead to exacerbations and persistent symptoms. Our IDO-ablated mice displayed those features by producing a decreased pattern of OVA-specific IgE and OVA-specific IgG1, even with mitigated Th2 asthmatic features, following repeated allergen and hRV exposure.
To the best of our knowledge, this study is the first to demonstrate that IDO is predisposed to attenuate Th17- and Th1-type neutrophilic airway inflammation in the course of asthma exacerbations caused by hRV infection. Importantly, our data reveal that IDO enzymatic induction in HSC-derived leukocytes plays a pivotal role in attenuating neutrophilic airway inflammation. Further studies are needed to elucidate the possible mechanisms of TRP metabolites and Th17/Treg balance in regulating Th17- and Th1-type neutrophilic airway inflammation during asthma exacerbations caused by hRV infection.
ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through National Research Foundation of Korea (NRF) grants funded by the Ministry of Education and the Ministry of Science and ICT (MSIT), Republic of Korea (2021R1A2B5B02001578, 2019R1A6A1A03033084, http://www.nrf.re.kr for Seong Kug Eo and 2020R1A6A3A13069626, http://www.nrf.re.kr for Seong Ok Park). The funder had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
We thank Dr. Yoon-Young Choi, Center for University Research Facility (CURF) at Chonbuk National University for providing technical assistance for the FACS analysis.
Abbreviations
- APC
allophycocyanin
- BALF
bronchoalveolar lavage fluid
- BM
bone marrow
- hRV
human rhinovirus
- DC
dendritic cell
- dpi
days post-infection
- HSC
hematopoietic stem cell
- ICS
inhaled corticosteroid
- IDO
indoleamine 2,3-dioxygenase
- i.n.
intranasal
- i.p.
intraperitoneal
- KYN
kynurenine
- mLN
mediastinal lymph node
- OVA
ovalbumin
- PAS
periodic acid-Schiff
- pDC
plasmacytoid dendritic cell
- RT
room temperature
- RSV
respiratory syncytial virus
- TJs
tight junctions
- TRP
tryptophan
- WT
wild-type
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Hossain FMA, Park SO, Eo SK.
- Data curation: Hossain FMA, Park SO.
- Formal analysis: Park SO, Choi JY, Eo SK.
- Funding acquisition: Eo SK.
- Investigation: Park SO, Kim HJ, Eo JC, Tanveer M, Uyangaa E, Eo SK.
- Methodology: Hossain FMA, Park SO, Kim HJ, Eo JC, Choi JY, Tanveer M, Uyangaa E, Eo SK.
- Project administration: Eo SK.
- Resources: Kim K.
- Validation: Choi JY, Uyangaa E, Kim K.
- Writing - original draft: Hossain FMA, Park SO, Eo SK.
- Writing - review & editing: Eo SK.
References
- 1.Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42:5–15. doi: 10.1007/s00281-020-00785-1. [DOI] [PubMed] [Google Scholar]
- 2.Kim YM, Kim YS, Jeon SG, Kim YK. Immunopathogenesis of allergic asthma: more than the th2 hypothesis. Allergy Asthma Immunol Res. 2013;5:189–196. doi: 10.4168/aair.2013.5.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hossain FMA, Choi JY, Uyangaa E, Park SO, Eo SK. The interplay between host immunity and respiratory viral infection in asthma exacerbation. Immune Netw. 2019;19:e31. doi: 10.4110/in.2019.19.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jartti T, Bønnelykke K, Elenius V, Feleszko W. Role of viruses in asthma. Semin Immunopathol. 2020;42:61–74. doi: 10.1007/s00281-020-00781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castillo JR, Peters SP, Busse WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract. 2017;5:918–927. doi: 10.1016/j.jaip.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinke JW, Borish L. Immune responses in rhinovirus-induced asthma exacerbations. Curr Allergy Asthma Rep. 2016;16:78. doi: 10.1007/s11882-016-0661-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. Rhinovirus-induced alterations on peripheral blood mononuclear cell phenotype and costimulatory molecule expression in normal and atopic asthmatic subjects. Clin Exp Allergy. 2002;32:537–542. doi: 10.1046/j.0954-7894.2002.01313.x. [DOI] [PubMed] [Google Scholar]
- 8.Gehlhar K, Bilitewski C, Reinitz-Rademacher K, Rohde G, Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006;36:331–337. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 9.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Jr, Calatroni A, Wildfire JJ, Gergen PJ, Cohen RT, Pongracic JA, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–1485. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, Calabrese F, Caramori G, Ballarin A, Snijders D, et al. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130:1307–1314. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Turner RB, Weingand KW, Yeh CH, Leedy DW. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin Infect Dis. 1998;26:840–846. doi: 10.1086/513922. [DOI] [PubMed] [Google Scholar]
- 14.Proud D, Turner RB, Winther B, Wiehler S, Tiesman JP, Reichling TD, Juhlin KD, Fulmer AW, Ho BY, Walanski AA, et al. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am J Respir Crit Care Med. 2008;178:962–968. doi: 10.1164/rccm.200805-670OC. [DOI] [PubMed] [Google Scholar]
- 15.Muehling LM, Heymann PW, Wright PW, Eccles JD, Agrawal R, Carper HT, Murphy DD, Workman LJ, Word CR, Ratcliffe SJ, et al. Human TH1 and TH2 cells targeting rhinovirus and allergen coordinately promote allergic asthma. J Allergy Clin Immunol. 2020;146:555–570. doi: 10.1016/j.jaci.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Han M, Ishikawa T, Bermick JR, Rajput C, Lei J, Goldsmith AM, Jarman CR, Lee J, Bentley JK, Hershenson MB. IL-1β prevents ILC2 expansion, type 2 cytokine secretion, and mucus metaplasia in response to early-life rhinovirus infection in mice. Allergy. 2020;75:2005–2019. doi: 10.1111/all.14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Southworth T, Pattwell C, Khan N, Mowbray SF, Strieter RM, Erpenbeck VJ, Singh D. Increased type 2 inflammation post rhinovirus infection in patients with moderate asthma. Cytokine. 2020;125:154857. doi: 10.1016/j.cyto.2019.154857. [DOI] [PubMed] [Google Scholar]
- 19.Basnet S, Bochkov YA, Brockman-Schneider RA, Kuipers I, Aesif SW, Jackson DJ, Lemanske RF, Jr, Ober C, Palmenberg AC, Gern JE. CDHR3 asthma-risk genotype affects susceptibility of airway epithelium to rhinovirus C infections. Am J Respir Cell Mol Biol. 2019;61:450–458. doi: 10.1165/rcmb.2018-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shigemasa R, Masuko H, Hyodo K, Kitazawa H, Kanazawa J, Yatagai Y, Iijima H, Naito T, Saito T, Hirota T, et al. Genetic impact of CDHR3 on the adult onset of asthma and COPD. Clin Exp Allergy. 2020;50:1223–1229. doi: 10.1111/cea.13699. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung AW, Terentis AC, King NJ, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci (Lond) 2015;129:601–672. doi: 10.1042/CS20140392. [DOI] [PubMed] [Google Scholar]
- 23.Mellor AL, Lemos H, Huang L. Indoleamine 2,3-dioxygenase and tolerance: Where are we now? Front Immunol. 2017;8:1360. doi: 10.3389/fimmu.2017.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gostner JM, Becker K, Kofler H, Strasser B, Fuchs D. Tryptophan metabolism in allergic disorders. Int Arch Allergy Immunol. 2016;169:203–215. doi: 10.1159/000445500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Leek AP, Yanishevsky Y, Kozyrskyj AL. The kynurenine pathway as a novel link between allergy and the gut microbiome. Front Immunol. 2017;8:1374. doi: 10.3389/fimmu.2017.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 27.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 28.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett. 2008;121:1–6. doi: 10.1016/j.imlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Ghahary A, Moqbel R. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Oriss TB, Fei M, Henry AC, Melgert BN, Chen L, Mellor AL, Munn DH, Irvin CG, Ray P, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci U S A. 2008;105:6690–6695. doi: 10.1073/pnas.0708809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, Broide DH, Carson DA, Raz E. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest. 2004;114:270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luukkainen A, Karjalainen J, Hurme M, Paavonen T, Huhtala H, Toppila-Salmi S. Relationships of indoleamine 2,3-dioxygenase activity and cofactors with asthma and nasal polyps. Am J Rhinol Allergy. 2014;28:e5–ee10. doi: 10.2500/ajra.2014.28.4013. [DOI] [PubMed] [Google Scholar]
- 34.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol. 2010;126:754–762.e1. doi: 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y, Chen Z, Zeng J, Zheng S, Sun L, Zhu L, Liao W. Th17/Treg imbalance is associated with reduced indoleamine 2,3 dioxygenase activity in childhood allergic asthma. Allergy Asthma Clin Immunol. 2020;16:61. doi: 10.1186/s13223-020-00457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin L, Hu Q, Hu Y, Chen Z, Liao W. Respiratory syncytial virus infection reduces kynurenic acid production and reverses Th17/Treg balance by modulating indoleamine 2,3-dioxygenase (IDO) molecules in plasmacytoid dendritic cells. Med Sci Monit. 2020;26:e926763. doi: 10.12659/MSM.926763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaelings L, Söderholm S, Bugai A, Fu Y, Nandania J, Schepens B, Lorey MB, Tynell J, Vande Ginste L, Le Goffic R, et al. Regulation of kynurenine biosynthesis during influenza virus infection. FEBS J. 2017;284:222–236. doi: 10.1111/febs.13966. [DOI] [PubMed] [Google Scholar]
- 38.van der Sluijs KF, van de Pol MA, Kulik W, Dijkhuis A, Smids BS, van Eijk HW, Karlas JA, Molenkamp R, Wolthers KC, Johnston SL, et al. Systemic tryptophan and kynurenine catabolite levels relate to severity of rhinovirus-induced asthma exacerbation: a prospective study with a parallel-group design. Thorax. 2013;68:1122–1130. doi: 10.1136/thoraxjnl-2013-203728. [DOI] [PubMed] [Google Scholar]
- 39.Hossain FMA, Park SO, Kim HJ, Eo JC, Choi JY, Uyangaa E, Kim B, Kim K, Eo SK. CCR5 attenuates neutrophilic airway inflammation exacerbated by infection with rhinovirus. Cell Immunol. 2020;351:104066. doi: 10.1016/j.cellimm.2020.104066. [DOI] [PubMed] [Google Scholar]
- 40.Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009;9:418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Reber LL, Daubeuf F, Nemska S, Frossard N. The AGC kinase inhibitor H89 attenuates airway inflammation in mouse models of asthma. PLoS One. 2012;7:e49512. doi: 10.1371/journal.pone.0049512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni ZH, Tang JH, Chen G, Lai YM, Chen QG, Li Z, Yang W, Luo XM, Wang XB. Resveratrol inhibits mucus overproduction and MUC5AC expression in a murine model of asthma. Mol Med Rep. 2016;13:287–294. doi: 10.3892/mmr.2015.4520. [DOI] [PubMed] [Google Scholar]
- 43.Kubo F, Ariestanti DM, Oki S, Fukuzawa T, Demizu R, Sato T, Sabirin RM, Hirose S, Nakamura N. Loss of the adhesion G-protein coupled receptor ADGRF5 in mice induces airway inflammation and the expression of CCL2 in lung endothelial cells. Respir Res. 2019;20:11. doi: 10.1186/s12931-019-0973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peake K, Manning J, Lewis CA, Barr C, Rossi F, Krieger C. Busulfan as a myelosuppressive agent for generating stable high-level bone marrow chimerism in mice. J Vis Exp. 2015:e52553. doi: 10.3791/52553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calkins CM, Barsness K, Bensard DD, Vasquez-Torres A, Raeburn CD, Meng X, McIntyre RC., Jr Toll-like receptor-4 signaling mediates pulmonary neutrophil sequestration in response to gram-positive bacterial enterotoxin. J Surg Res. 2002;104:124–130. doi: 10.1006/jsre.2002.6422. [DOI] [PubMed] [Google Scholar]
- 46.Looi K, Buckley AG, Rigby PJ, Garratt LW, Iosifidis T, Zosky GR, Larcombe AN, Lannigan FJ, Ling KM, Martinovich KM, et al. Effects of human rhinovirus on epithelial barrier integrity and function in children with asthma. Clin Exp Allergy. 2018;48:513–524. doi: 10.1111/cea.13097. [DOI] [PubMed] [Google Scholar]
- 47.Tan HT, Hagner S, Ruchti F, Radzikowska U, Tan G, Altunbulakli C, Eljaszewicz A, Moniuszko M, Akdis M, Akdis CA, et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy. 2019;74:294–307. doi: 10.1111/all.13619. [DOI] [PubMed] [Google Scholar]
- 48.Yeo NK, Jang YJ. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope. 2010;120:346–352. doi: 10.1002/lary.20764. [DOI] [PubMed] [Google Scholar]
- 49.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams EJ, Negewo NA, Baines KJ. Role of the NLRP3 inflammasome in asthma: Relationship with neutrophilic inflammation, obesity, and therapeutic options. J Allergy Clin Immunol. 2021;147:2060–2062. doi: 10.1016/j.jaci.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 51.Misra RS. A review of the CD4+ T cell contribution to lung infection, inflammation and repair with a focus on wheeze and asthma in the pediatric population. EC Microbiol. 2014;1:4–14. [PMC free article] [PubMed] [Google Scholar]
- 52.Platts-Mills TA. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001;164:S1–S5. doi: 10.1164/ajrccm.164.supplement_1.2103024. [DOI] [PubMed] [Google Scholar]
- 53.Alrifai M, Marsh LM, Dicke T, Kılıç A, Conrad ML, Renz H, Garn H. Compartmental and temporal dynamics of chronic inflammation and airway remodelling in a chronic asthma mouse model. PLoS One. 2014;9:e85839. doi: 10.1371/journal.pone.0085839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan J, Yang Q, Zhou Y, Deng H, Zhu Y, Zhao D, Liu F. MicroRNA-221 modulates airway remodeling via the PI3K/AKT pathway in ova-induced chronic murine asthma. Front Cell Dev Biol. 2020;8:495. doi: 10.3389/fcell.2020.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu H, Gong J, Liu Y. Indoleamine 2, 3-dioxygenase regulation of immune response (Review) Mol Med Rep. 2018;17:4867–4873. doi: 10.3892/mmr.2018.8537. [DOI] [PubMed] [Google Scholar]
- 56.Nicoli F, Paul S, Appay V. Harnessing the induction of CD8+ T-cell responses through metabolic regulation by pathogen-recognition-receptor triggering in antigen presenting cells. Front Immunol. 2018;9:2372. doi: 10.3389/fimmu.2018.02372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han M, Rajput C, Ishikawa T, Jarman CR, Lee J, Hershenson MB. Small animal models of respiratory viral infection related to asthma. Viruses. 2018;10:682. doi: 10.3390/v10120682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokulsky LA, Garcia-Netto K, Nguyen TH, Girkin JLN, Collison A, Mattes J, Kaiko G, Liu C, Bartlett NW, Yang M, et al. A critical role for the CXCL3/CXCL5/CXCR2 neutrophilic chemotactic axis in the regulation of type 2 responses in a model of rhinoviral-induced asthma exacerbation. J Immunol. 2020;205:2468–2478. doi: 10.4049/jimmunol.1901350. [DOI] [PubMed] [Google Scholar]
- 59.von Bubnoff D, Bieber T. The indoleamine 2,3-dioxygenase (IDO) pathway controls allergy. Allergy. 2012;67:718–725. doi: 10.1111/j.1398-9995.2012.02830.x. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, Elliott GI, Kufareva I, Abagyan R, Broide DH, et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci U S A. 2007;104:18619–18624. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taher YA, Piavaux BJ, Gras R, van Esch BC, Hofman GA, Bloksma N, Henricks PA, van Oosterhout AJ. Indoleamine 2,3-dioxygenase-dependent tryptophan metabolites contribute to tolerance induction during allergen immunotherapy in a mouse model. J Allergy Clin Immunol. 2008;121:983–991.e2. doi: 10.1016/j.jaci.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Kim KW, Kim HR, Kim BM, Won JY, Lee KA, Lee SH. Intravenous immunoglobulin controls Th17 cell-mediated osteoclastogenesis. Immune Netw. 2019;19:e27. doi: 10.4110/in.2019.19.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, Cullimore ML, Rostami A, Xu H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moon J, Lee SH, Lee SY, Ryu J, Jhun J, Choi J, Kim GN, Roh S, Park SH, Cho ML. GRIM-19 ameliorates multiple sclerosis in a mouse model of experimental autoimmune encephalomyelitis with reciprocal regualtion of IFNγ/Th1 and IL-17A/Th17 cells. Immune Netw. 2020;20:e40. doi: 10.4110/in.2020.20.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van der Sluijs K, Singh R, Dijkhuis A, Snoek M, Lutter R. Indoleamine-2,3-dioxygenase activity induces neutrophil apoptpsis. Crit Care. 2011;15:208. [Google Scholar]
- 66.Loughman JA, Hunstad DA. Induction of indoleamine 2,3-dioxygenase by uropathogenic bacteria attenuates innate responses to epithelial infection. J Infect Dis. 2012;205:1830–1839. doi: 10.1093/infdis/jis280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeon JH, Hong CW, Kim EY, Lee JM. Current understanding on the metabolism of neutrophils. Immune Netw. 2020;20:e46. doi: 10.4110/in.2020.20.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar RK, Herbert C, Foster PS. The “classical” ovalbumin challenge model of asthma in mice. Curr Drug Targets. 2008;9:485–494. doi: 10.2174/138945008784533561. [DOI] [PubMed] [Google Scholar]
- 69.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178–1187. doi: 10.1016/j.jaci.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng J, Yu XQ, Wang PH. Inflammasome activation and Th17 responses. Mol Immunol. 2019;107:142–164. doi: 10.1016/j.molimm.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 71.Guan M, Ma H, Fan X, Chen X, Miao M, Wu H. Dexamethasone alleviate allergic airway inflammation in mice by inhibiting the activation of NLRP3 inflammasome. Int Immunopharmacol. 2020;78:106017. doi: 10.1016/j.intimp.2019.106017. [DOI] [PubMed] [Google Scholar]
- 72.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 73.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan HT, Hagner S, Ruchti F, Radzikowska U, Tan G, Altunbulakli C, Eljaszewicz A, Moniuszko M, Akdis M, Akdis CA, et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy. 2019;74:294–307. doi: 10.1111/all.13619. [DOI] [PubMed] [Google Scholar]