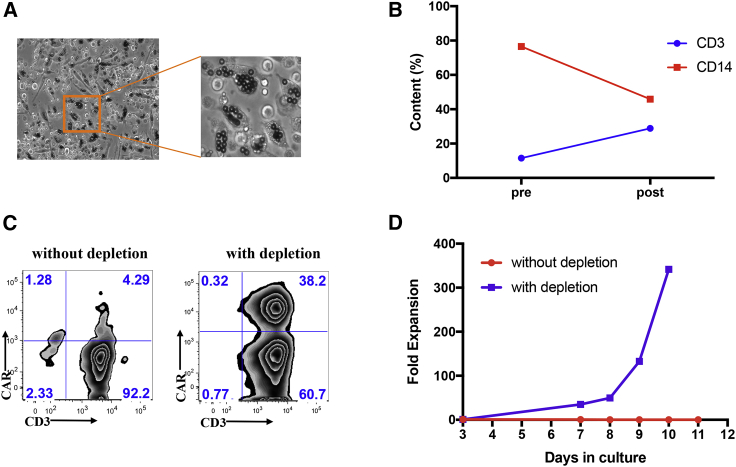
Figure 1.
Removal of CD14+ cells by plastic adhesion rescues large-scale clinical CAR T manufacturing for an ALL patient (ClinicalTrials.gov: NCT01044069)
(A) Microscopic observation of Dynabeads uptaken by cells adhering to PermaLife bag 3 days after selection and activation during CAR T cell manufacturing run (without CD14+ cell removal by plastic adhesion). (B) Percentage of CD3+ (blue) and CD14+ (red) contents prior to and after two-step, 1.5 h and overnight, plastic adhesion monocyte depletion procedure. (C) Transduction efficiency of CAR T cells for manufacturing runs without (left) or with (right) monocyte depletion procedure. (D) Cumulative fold expansion of total viable cells for the large-scale manufacturing runs with (blue) or without (red) monocyte depletion step since the day of transduction.