Figure 4.
Pre- and post-monocyte depletion percentage of CD14+ and CD3+ cells, fold expansion and total number of CAR T cells by the end of production runs following CD14+ cell removal
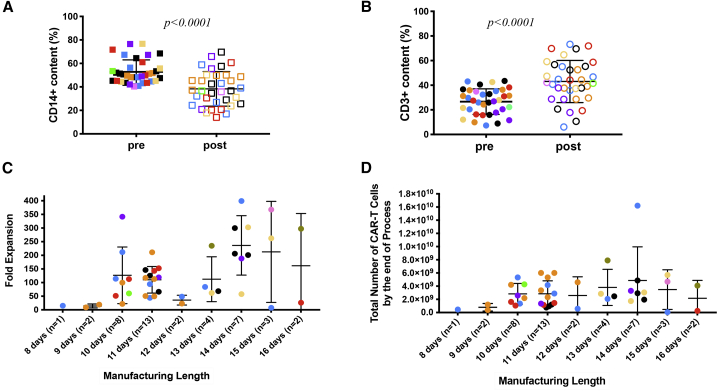
(A) A 2-h plastic adhesion monocyte depletion step significantly altered the percentage of CD14+ and CD3+ cells in apheresis products with ≥40% CD14+ monocytes. A total of 35 apheresis products with originally ≥40% CD14+ monocytes from different disease indications (CLL: ivory [ClinicalTrials.gov: NCT00466531] and orange [ClinicalTrials.gov: NCT03085173]; ALL: dark purple; pALL: green; NHL: red; meso: blue; OVA: tan; TNB: light purple; and MM: black) were monitored. Means ± SEM of the percentage of CD14+ cell content pre- and post-plastic adhesion procedure are shown and compared using Student’s t test, p < 0.0001. Solid squares represent % CD14 cells in suspension pre-attachment, and empty squares represent remaining % CD14 cells in suspension post-attachment. (B) Means ± SEM of the percentage of CD3+ cells pre- and post-plastic adhesion procedure in the same apheresis products as in (A) are shown and compared using Student’s t test, p < 0.0001. The solid circles represent the % CD3+ cells in suspension pre-attachment, and the empty circles represent the % CD3+ cells in suspension post-attachment. (C and D) Fold expansion (C) and total number of CAR T cells obtained at the end of each manufacturing process (D), plotted according to length of manufacturing for 42 manufacturing runs derived from apheresis products with initial CD14+ monocyte content ≥40% and that underwent 2-h plastic adherence step. Different colors represent different disease indications as specified in (A).