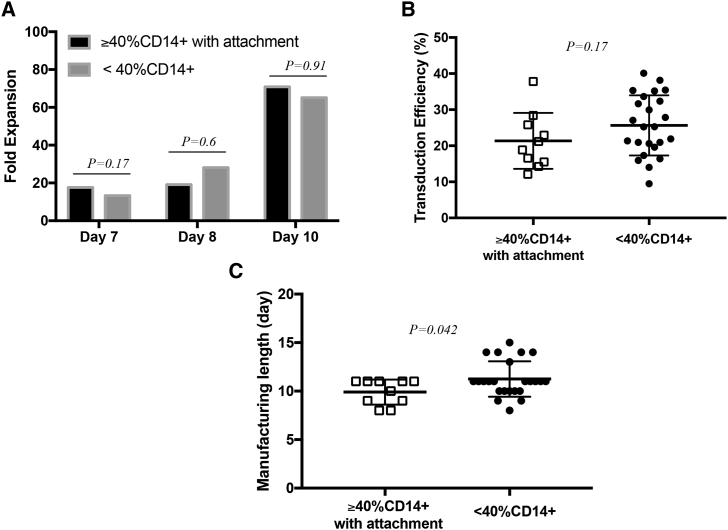
Figure 6.
Case study of CAR T cell manufacturing runs for patients with relapsed/refractory CLL (ClinicalTrials.gov: NCT03085173; n = 34)
(A) Comparison of cumulative fold expansion of total viable cells between CAR T cell manufacturing runs started with apheresis products containing initially ≥40% CD14+ monocytes that underwent a 2-h plastic adhesion monocyte depletion step (n = 10), and the manufacturing runs started with apheresis products containing initially <40% CD14+ monocytes (n = 24). Average fold expansion is shown and compared using Student’s t test. At day 7, p = 0.17; at day 8, p = 0.6; and at day 10, p = 0.91. (B) Comparison of transduction efficiency between CAR T cell manufacturing runs started with apheresis products containing initially ≥40% CD14+ monocytes that underwent a 2-h plastic adhesion monocyte depletion step (n = 10, open squares) and shown for each group, and the groups were compared using Student’s t test, p = 0.17. (C) Comparison of manufacturing length between CAR T cell manufacturing runs started with apheresis products containing initially ≥40% CD14+ monocytes that underwent a 2-h plastic adhesion monocyte depletion step (n = 10, open squares), and the manufacturing runs started with apheresis products containing initially <40% CD14+ monocytes (n=24, solid circles). Average manufacturing length ± SEM is shown for each group, and the groups were compared using Student’s t-test, p = 0.042.