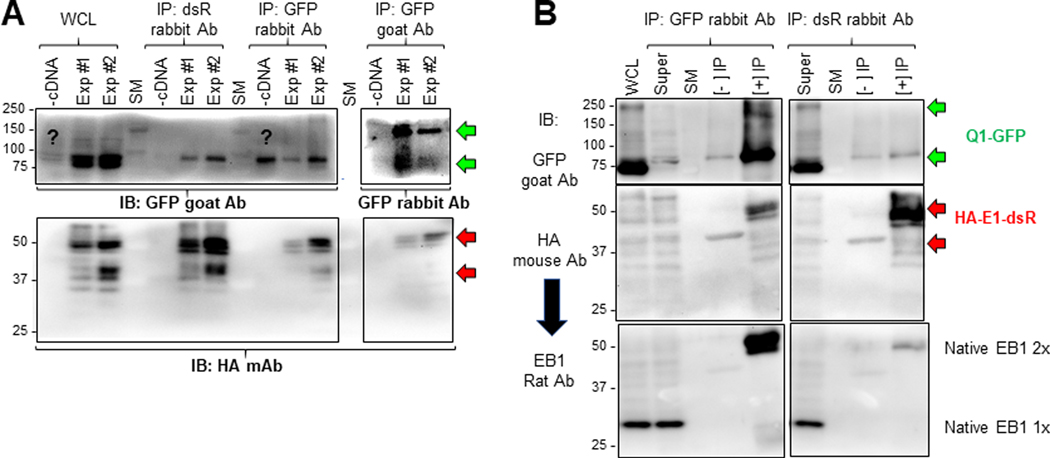
Fig. 11. Check the quality of immunoprecipitates from HEK293 cells before LC/MS-MS, and validate a novel Q1-GFP interactor (microtubule plus-end binding protein 1, EB1) identified in proteomic experiments.
Two batches of HEK293 cells (Exp #1 and Exp #2) transfected with Q1-GFP and HA-E1-dsR, and one batch of untransfected HEK293 cells (-cDNA) were lysed in 1% Triton X-100 lysis buffer. Three equal fractions of WCL from each of the three were subject to immunoprecipitation with dsR rabbit Ab, GFP rabbit Ab and GFP goat Ab. (A) Immunoblot analysis of WCL and immunoprecipitates. IP lanes were loaded 5% of the total immunoprecipitates. After SDS-PAGE and protein transfer to PVDF membrane, the membrane was cut below the 75 kDa size marker (SM) band. The upper portion was probed with GFP goat Ab (WCL and IP with rabbit Abs) or GFP rabbit Ab (IP with GFP goat Ab). The lower portion was probed with HA mouse Ab. (B) Test native EB1 protein in immunoprecipitates with GFP rabbit Ab (left) and dsR rabbit Ab (right). WCL and three lanes for each IP reaction (Super = WCL after IP, [-] IP without Ab, [+] IP with Ab) were fractionated by SDS-PAGE. After proteins were transferred to PVDF membrane, the membrane was cut below 75 kDa size marker band. The upper portion was probed with GFP goat Ab. The lower portion was first probed with HA mouse Ab, and after stripping, reprobed with EB1 rat Ab. The green and red arrows point to expected Q1-GFP bands (100 kDa and 200 kDa, monomer and dimer) and HA-E1-dsR bands (42 kDa if unglycosylated, higher molecular weights if N- and/or O-glycosylated (Chandrasekhar et al., 2011)). Native EB1 migrated as monomer and dimer (30 and 60 kDa, respectively) (Chen et al., 2014).