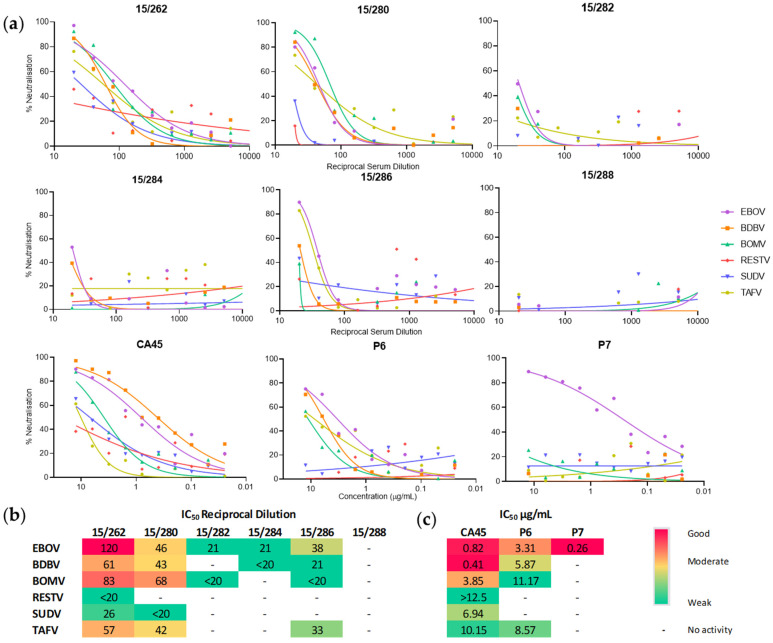
Figure 5.
Cross-neutralisation of BOMV afforded by a panel of human EBOV convalescent serum and monoclonal antibodies. (a) Dose-response curves plotted as percentage neutralisation for dilutions of the WHO IS (15/262) and WHO Reference Panel members from four individuals recovered from EBOV (15/280, 15/282, 15/284, 15/286) and a negative control (15/288) along with two pan-ebolavirus mAbs (CA45, P6) and an EBOV-specific mAb negative control (P7). (n = 2) (b,c) 50% inhibitory concentrations (IC50) expressed as reciprocal of the dilution factor for plasma samples (b) or in μg/mL for mAb (c); IC50 were interpolated via non-linear regression analysis, with potency ranked good to weak based on a 50th percentile mid-point. Where the IC50 is outside the dilution range tested, values are reported as greater or less than the minimum serum (1:20) (b) and mAb (12.5 μg/mL) (c) dilution tested.