Figure 1 |. Summary of photosensitizer administration routes for photodynamic therapy of cancer in clinical trials that are published by the U.S. National Library of Medicine.
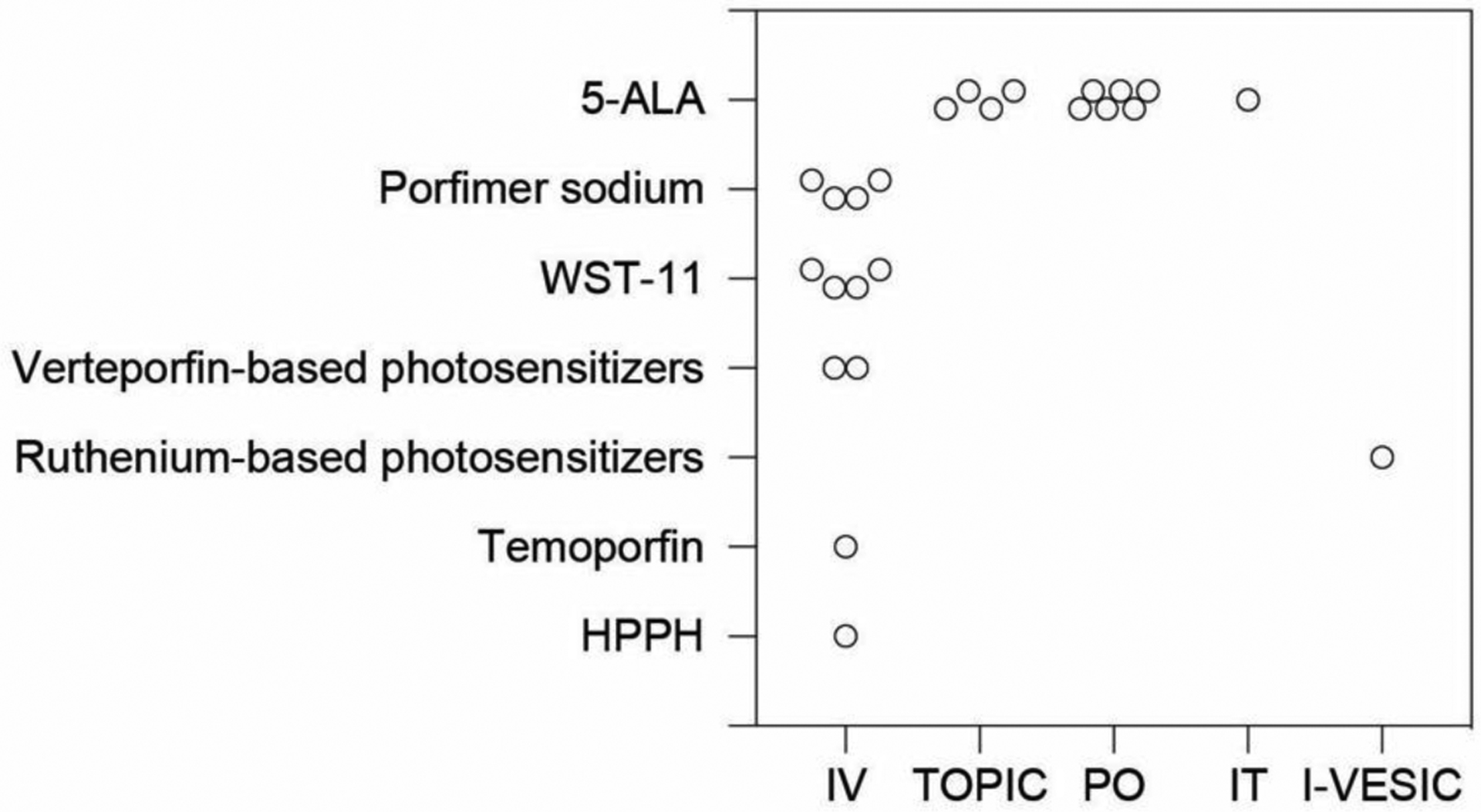
Data collected from the clinicaltrials.gov website show different photosensitizers (y-axis) and their respective administration routes (x-axis). Each dot in the plot represents a clinical trial that is Active (not recruiting), Enrolling by invitation, Recruiting, or Not yet recruiting. Our summary indicates a majority of photosensitizers are currently administered intravenously in the clinic, with the exception of 5-ALA. In the plot, clinical trials that are Suspended, Terminated, Completed, Withdrawn, or have Unknown status were not included. Photosensitizers include 5-aminolevulinic acid (5-ALA or Gliolan®), porfimer sodium (Photofrin®), WST-11 (TOOKAD® Soluble), verteporfin-based PS (e.g., Visudyne®, HS-201), ruthenium-based PS (TLD1433), temoporfin (tetra[m-hydroxyphenyl]chlorin, m-THPC, or Foscan®), and 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH or Photochlor®). Routes of administration include intravenous (IV, administration within or into a vein or veins), topical (TOPIC, administration to a particular spot on the outer surface of the body), oral (PO, administration to or by way of the mouth), intratumor (IT, administration within a tumor), and intravesical (I-VESIC, administration within the bladder).
