Figure 2 |. Chemical structures of clinically used photosensitizers described in Figure 1.
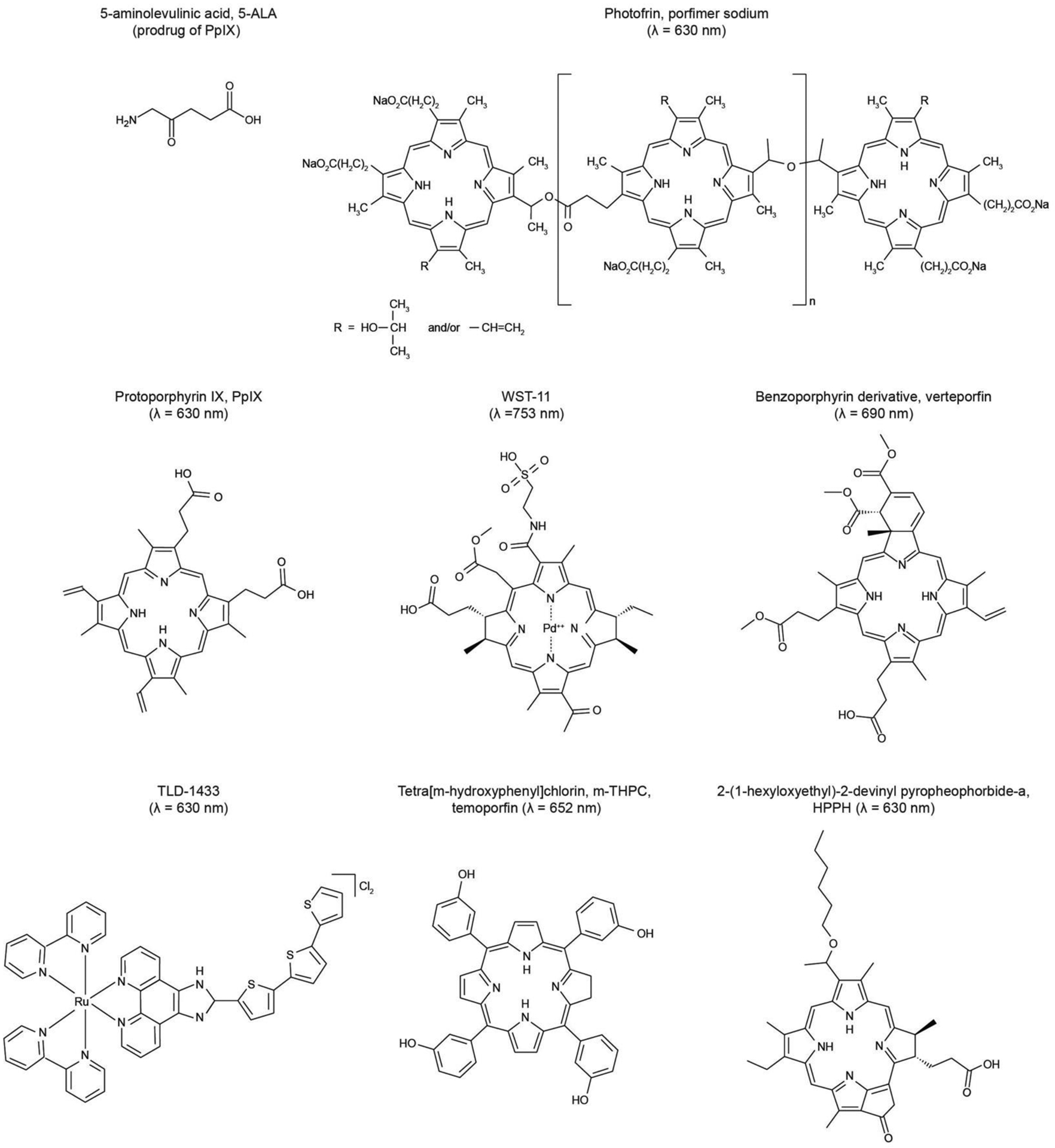
All the listed photosensitizers, besides the ruthenium-based photosensitizer TLD-1422, contain the tetrapyrrole macrocycle structure. The porphyrin-based photosensitizers (e.g., Photofrin, protoporphyrin IX) typically have a ring structure with 22 π-electrons, while the chlorin-type photosensitizer (i.e., tetra[m-hydroxyphenyl]chlorin) has one reduced double bond. These photosensitizers absorb light at red and near-infrared wavelengths, allowing for maximum penetration of light through tissues.
