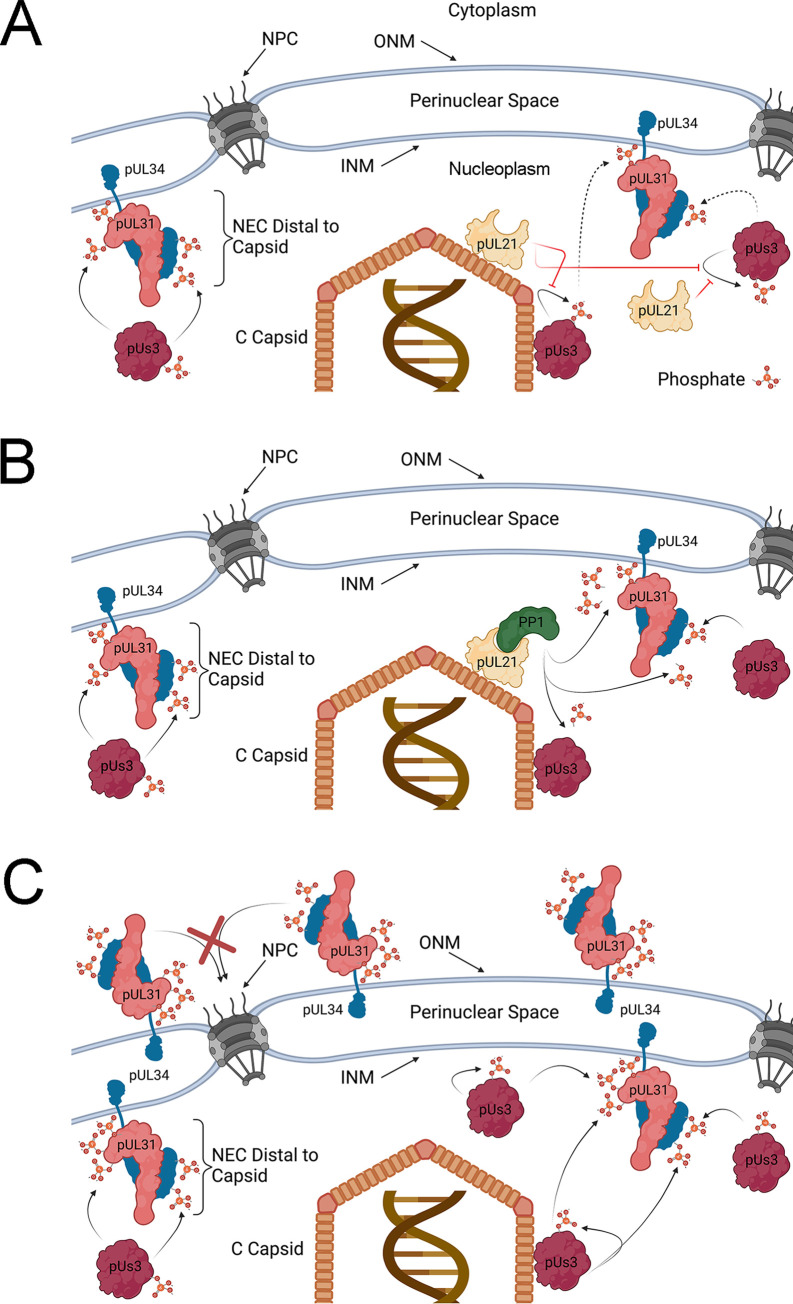
Fig 9. Models for regulation of HSV-2 NEC phosphorylation by pUL21.
(A) C-capsid associated pUL21 prevents the autophosphorylation of pUs3 (red lines) leading to reduced pUs3 kinase activity (dashed arrows), low level phosphorylation of capsid-proximal pUL31 and pUL34 and corresponding enhancement of NEC primary envelopment and INM invagination activity. Capsid distal NECs are not subject to pUL21 inhibition of pUs3 autophosphorylation and kinase activation, and so, these NECs are more heavily phosphorylated and lack NEC envelopment activity. Note that the phosphorylation of capsid-associated pUL31 (not shown) would also be expected to be subject to regulation by pUL21. (B) C-capsid associated pUL21 in complex with PP1 facilitate the dephosphorylation of capsid-proximal pUL31, pUL34 and pUs3 leading to enhancement of NEC primary envelopment and INM invagination activity. Capsid distal NEC components are not subject to pUL21/PP1 mediated dephosphorylation and thus these NECs are more heavily phosphorylated and lack NEC envelopment activity. Note that the phosphorylation of capsid-associated pUL31 (not shown) would also be expected to be subject to regulation by pUL21/PP1. Models A and B are not mutually exclusive and may operate in concert. (C) In the absence of pUL21, pUs3 kinase activity is unrestricted and dephosphorylation of pUL31, pUL34 and pUs3 is inefficient leading to hyperphosphorylation of the NEC and inhibition of NEC primary envelopment activity. Hyperphosphorylation of pUL31 interferes with the nuclear import of pUL31/pUL34 NEC complexes in the ONM (red X). Resulting accumulation of hyperphosphorylated NECs in the ONM may lead to nuclear envelope extravagations seen in the absence of pUL21. Outer nuclear membrane (ONM), inner nuclear membrane (INM), nuclear pore complex (NPC). Figure created with BioRender.com.