Figure 2.
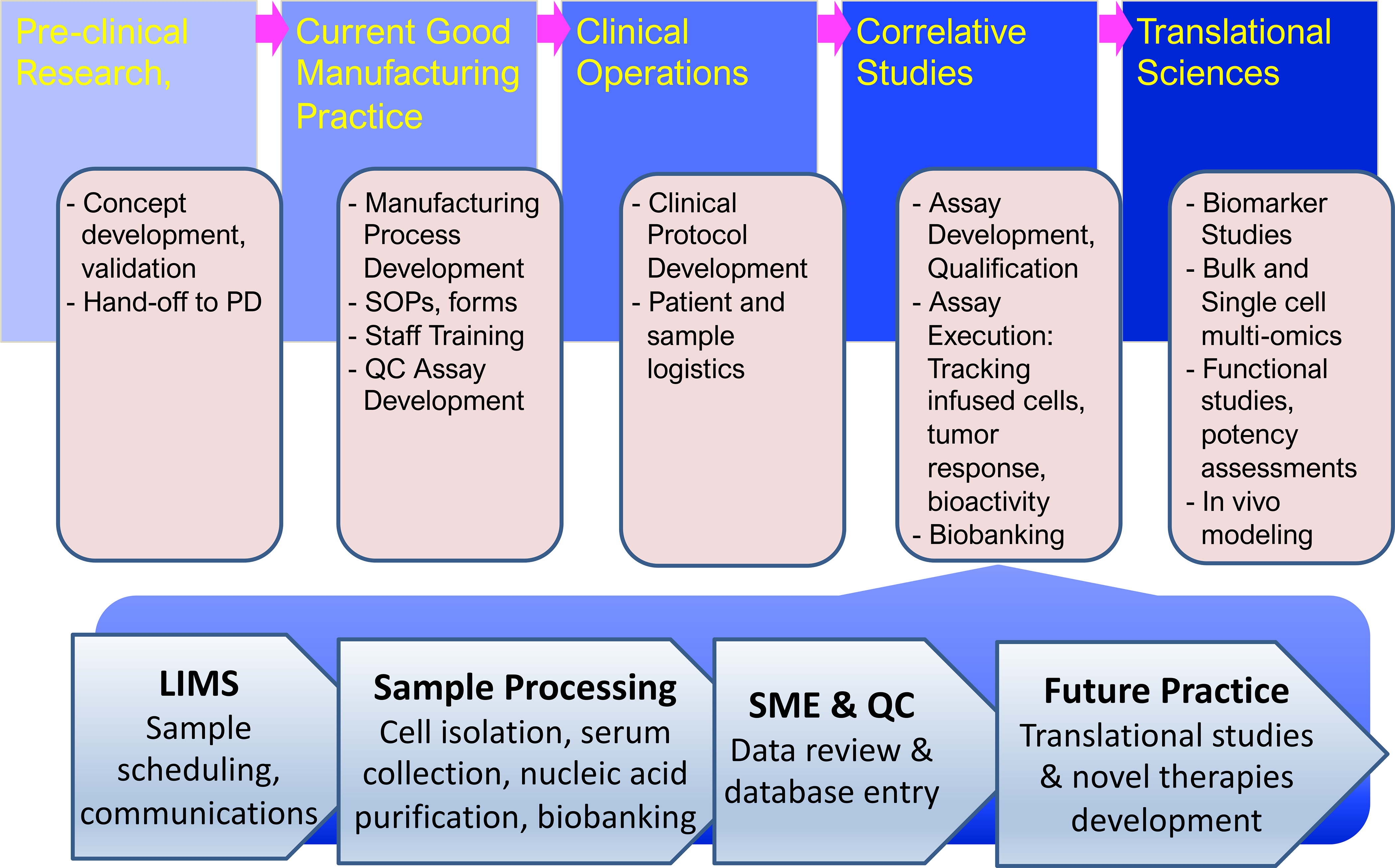
Operational pipeline for integrating correlative studies in translational science laboratories. Novel therapies developed and pre-clinically validated in research laboratories are handed off to the process development (PD) team for scale-up and the development of a current Good Manufacturing Practice (GMP) process. In collaboration with the GMP teams Standard Operating Procedures (SOP) and documentation forms are developed and GMP staff trained in the new procedures. The Correlative Studies Laboratory will, in parallel, ensure that all supportive assays, protocols, and forms are in place, that staff is trained, and that routine, qualified assays are developed and biobanking ensured. This same team is also involved in protocol development, which is lead by the Clinical Operations team with feedback from the study clinicians and the research laboratory that developed the new process. When a new clinical trial begins the Correlative Studies laboratory starts receiving biospecimens from the clinic, manufacturing facility, or collaborating laboratories, and logs these samples into the Laboratory Information Management System (LIMS), to be processed as specified by standard operating procedures and examined using validated assays by qualified personnel. Aliquots are retained from each specimen for future translational studies. The data are reviewed by subject matter experts (SME) before being reviewed by the quality control (QC) manager and entered into a database. A staff statistician cleans and analyzes the data for reporting purposes, e.g. to FDA or for scientific meetings and manuscript preparation.
