Abstract
Background
Racial and ethnic minority groups have been disproportionately affected by the US coronavirus disease 2019 (COVID-19) pandemic; however, nationwide data on COVID-19 outcomes stratified by race/ethnicity and adjusted for clinical characteristics are sparse. This study analyzed the impacts of race/ethnicity on outcomes among US patients with COVID-19.
Methods
This was a retrospective observational study of patients with a confirmed COVID-19 diagnosis in the electronic health record from 01 February 2020 through 14 September 2020. Index encounter site, hospitalization, and mortality were assessed by race/ethnicity (Hispanic, non-Hispanic Black [Black], non-Hispanic White [White], non-Hispanic Asian [Asian], or Other/unknown). Associations between racial/ethnic categories and study outcomes adjusted for patient characteristics were evaluated using logistic regression.
Findings
Among 202,908 patients with confirmed COVID-19, patients from racial/ethnic minority groups were more likely than White patients to be hospitalized on initial presentation (Hispanic: adjusted odds ratio 1·690, 95% CI 1·620–1·763; Black: 1·810, 1·743–1·880; Asian: 1·503, 1·381–1·636) and during follow-up (Hispanic: 1·700, 1·638–1·764; Black: 1·578, 1·526–1·633; Asian: 1·391, 1·288–1·501). Among hospitalized patients, adjusted mortality risk was lower for Black patients (0·881, 0·809–0·959) but higher for Asian patients (1·205, 1·000–1·452).
Interpretation
Racial/ethnic minority patients with COVID-19 had more severe disease on initial presentation than White patients. Increased mortality risk was attenuated by hospitalization among Black patients but not Asian patients, indicating that outcome disparities may be mediated by distinct factors for different groups. In addition to enacting policies to facilitate equitable access to COVID-19–related care, further analyses of disaggregated population-level COVID-19 data are needed.
Funding
None.
Keywords: COVID-19, SARS-CoV-2, Retrospective Studies, Electronic Health Records, Ethnic Groups, Minority Groups, United States
Research in context.
Evidence before this study
It is clear that racial and ethnic minority populations have been disproportionately affected by the coronavirus disease 2019 (COVID-19) pandemic in the US; however, most studies of racial/ethnic disparity in COVID-19 outcomes to date have not adjusted for comorbidities and other clinical characteristics, or have been limited to specific geographies or health systems. We searched PubMed in August 2020 using the string “rac* AND covid AND outcomes,” focusing on US studies in order to identify analyses that were most relevant to the patient population in our database. Although this search returned hundreds of results, closer investigation revealed no studies conducted across a nationwide population.
Added value of this study
Using information from a large electronic health record dataset consolidated from hospital systems and provider networks across the US, we found that patients from racial/ethnic minority groups were more likely than White patients to have their initial COVID-19–related healthcare encounter in a hospital setting and to require subsequent hospitalization after adjusting for demographic and clinical characteristics. Notably, adjustment for comorbidities moderately reduced follow-up mortality risk for Black patients, but not for Asian patients. Among those who had been hospitalized, adjusted mortality risk was lower among Black patients but remained elevated for Asian patients relative to White patients.
Implications of all the available evidence
Our findings suggest that racial/ethnic disparities in COVID-19 outcomes may be due in part to members of racial/ethnic minority groups who have asymptomatic or mild disease receiving less evaluation and testing and/or accessing treatment later in the disease process, missing the opportunity for early interventions that could slow or prevent disease progression. Together with existing evidence, our findings support the promotion of policy changes that facilitate timely access to COVID-19–related healthcare for racial and ethnic minority populations, such as improving access to COVID-19 vaccination, testing, and screening; conducting culturally appropriate outreach in underserved communities to emphasize the importance of vaccination and early medical attention for COVID-19; and ensuring that healthcare costs or fear of income loss are not barriers to receiving care.
Alt-text: Unlabelled box
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been devastating in the US, crippling the economy and overburdening the healthcare system [1,2]. As of November 30, 2020, there had been more than 13.9 million US cases of COVID-19 and over 280,000 deaths [3].
There is abundant evidence that racial and ethnic minority populations have been disproportionately affected by the pandemic. Over 60% of COVID-19 cases and nearly 50% of deaths in the US have occurred among patients in racial/ethnic minority groups; in particular, the mortality rate in the US Black population is more than twice that in the White population [3,4]. The existence of racial and ethnic disparities in COVID-19 morbidity and mortality is due in part to longstanding economic and educational disadvantages that have culminated in reduced healthcare utilization and prevalent mistrust of the healthcare system among minority individuals [5], [6], [7]. There also has been speculation that the increased vulnerability of racial/ethnic minority groups to COVID-19–related complications, especially among Black patients, is attributable to a higher burden of comorbidities—including hypertension, diabetes, kidney disease, and obesity—and/or other factors, such as pro-inflammatory changes in immune response and coagulation resulting from the psychosocial stress of living with systemic racism [8], [9], [10]. These factors are compounded by members of racial/ethnic minority groups being disproportionately likely to reside in areas of high population density, rely on public transportation, and work in public-facing “essential” occupations, making social distancing more difficult and increasing COVID-19 infection risk [11,12].
Until recently, nationwide analyses of COVID-19 morbidity and mortality stratified by race/ethnicity and adjusted for comorbidities have not been available. Many studies conducted thus far have relied on local and state health department data, either as collected on COVID-19 reporting websites or as aggregated by the US Centers for Disease Control and Prevention (CDC) [13], [14], [15], [16], [17]. Unfortunately, much of these data are at the population level rather than at the individual level, lack race/ethnicity information, and/or do not include clinical details necessary to assess confounding clinical factors. Other analyses have utilized data with comprehensive race/ethnicity and clinical information, but were limited to certain geographic areas or hospital systems [18], [19], [20], [21], [22], [23], [24], [25], [26]. In the present study, we analyzed the impacts of race/ethnicity and comorbidities on clinical outcomes among patients with confirmed COVID-19 using electronic health records (EHR) from a healthcare database representing over 31 million patients and spanning multiple payers and providers across the US.
2. Methods
2.1. Study design and data source
This was a retrospective observational study conducted using the Optum EHR Research Database, [27] a deidentified patient-level database that incorporates administrative and clinical data as recorded during routine clinical practice in both ambulatory and inpatient settings. Data are collected from tens of thousands of providers and hundreds of hospitals representing more than 50 provider/hospital networks across the US and include over 31 million lives, with patients covered by a variety of payers (including both commercial plans and government-administered plans such as Medicare/Medicaid) as well as uninsured patients.
Because the data used in this study were deidentified in accordance with the Health Insurance Portability and Accountability Act, [28] institutional review board approval or waiver of approval was not required. Authors Andrade, Johnson, Raja, Cao, and Hulbert had direct access to the database, and the data used for this analysis were available in November 2020.
2.2. Study sample
The study included all patients with a confirmed clinical COVID-19 diagnosis (ICD-10-CM U07.1) or positive COVID-19 polymerase chain reaction (PCR) or antigen test result in the EHR database during the identification period (01 February 2020 through 14 September 2020). The index date was defined as the earliest observed healthcare encounter associated with a confirmed COVID-19 diagnosis (including a positive COVID-19 PCR or antigen test; or diagnosis codes for confirmed or suspected COVID-19, coronavirus-related or severe acute respiratory syndrome [SARS]-related infection, or pneumonia due to coronavirus or SARS [Supplementary Table 1]). These criteria were developed in consideration of COVID-19 coding guidance provided by the CDC [29].
2.3. Study measures
Patient demographic characteristics, insurance type, and body mass index were assessed as of the index date; comorbidities and medication use were assessed during the baseline period, which began 13 months prior to the index date and ended 1 month prior to the index date. The month prior to the index date was excluded out of concern that it could reflect healthcare utilization associated with early effects of COVID-19 infection. Patient race/ethnicity (patient-reported or provider-documented) was identified from the EHR and categorized as Hispanic, non-Hispanic Black (Black), non-Hispanic Asian (Asian), non-Hispanic White (White), or Other/unknown. The Asian category includes patients identified as East Asian or Southeast Asian. The Other/unknown category includes patients with unknown race/ethnicity, those identified as Native Hawaiian/other Pacific Islander or American Indian/Alaskan Native, and those with multiple races reported (race/ethnicity was masked for these groups because sample sizes were too small to maintain the deidentified classification of the data source).
Study outcomes (index encounter site, all-cause hospitalization, and all-cause mortality) were assessed during the follow-up period, which began on the index date and ended on the last date of data availability (14 October 2020), with a minimum follow-up of 1 month. Index encounter site was defined as the highest level of healthcare intensity on the date of the index encounter (classified in mutually exclusive hierarchies of inpatient hospital admission, emergency department [ED] visit, ambulatory care visit, other visit [eg, laboratory, imaging, or report/result], or unknown/missing). In addition to index encounter site, an outcome of all-cause hospitalization during follow-up was defined as the first inpatient admission on the index date or during the follow-up period. All-cause mortality during the follow-up period was assessed from EHR (eg, hospital discharge status or medical record death indicator) and linked information from obituary data and the public-use Social Security Administration Death Master File.
2.4. Statistical analysis
All analyses were performed with SAS software version 9·4 (SAS Institute Inc). Patient characteristics and study outcomes were analyzed descriptively and stratified by race/ethnicity. Differences in patient characteristics and study outcomes across race/ethnicity categories were assessed using analysis of variance for continuous measures and Pearson chi-square tests for binary measures. Pairwise comparisons between racial/ethnic minority groups and White patients (referent group) were assessed using 2-sample t-tests. Statistical significance was defined as p ≤ 0·05.
The association between race/ethnicity categories and outcomes (index encounter site, follow-up hospitalization, and follow-up mortality) was evaluated using unconditional logistic regression. For each outcome, two multivariable models are presented: minimally adjusted (age group [0–44, 45–64, 65–74, 75–84, and 85+ years], sex, geographic region, insurance type, index month) and fully adjusted (age group, sex, geographic region, insurance type, index month, index body mass index, baseline Charlson comorbidity score, [30] baseline comorbidities, and baseline medication use) (Supplementary Tables 2–5). Comorbidity indicators and medications included in the fully adjusted models were selected on the basis of potential clinical relevance to COVID-19–related outcomes and statistical significance within the models using stepwise regression with a threshold of p < 0·10.
3. Results
3.1. Study sample
During the identification period, there were 2,014,956 patients with a COVID-19–related healthcare encounter or test in the EHR. Among these, 158,548 had a COVID-19 diagnosis code and 133,767 had a positive PCR or antigen test for COVID-19. As 89,158 patients met both criteria, this yielded a total of 202,908 unique patients with confirmed COVID-19 as the total study population (mean age 46·9 years, 55·3% women, 14·5% Hispanic, 15·6% Black, 2·6% Asian, 52·5% White, 14·9% Other/unknown) (Table 1). Among the 2,014,956 patients with a COVID-19–related healthcare encounter/test, the proportion with confirmed COVID-19 was 10·1% overall but varied significantly by race/ethnicity: Hispanic, 20·2%; Black, 15·2%; Asian, 11·9%; White, 7·8%; Other/unknown, 12·2% (p < 0·001) (data not shown).
Table 1.
Demographic and Clinical Characteristics of Patients With Confirmed COVID-19
| Characteristic | Totala N = 202,908 100.0% | Hispanic n = 29,326 14.5% | Non-Hispanic Black n = 31,547 15.6% | Non-Hispanic Asian n = 5,311 2.6% | Non-Hispanic White n = 106,547 52.5% | Other/unknownb n = 30,177 14.9% | Global p-valuec | Hisp. vs White p-valued | Black vs White p-valued | Asian vs White p-valued | Other vs White p-valued |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 46.9 (20.2) | 41.2 (18.2) | 47.3 (18.9) | 45.9 (18.1) | 49.6 (21.0) | 43 (19.1) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Age category, years, n (%) | |||||||||||
| 0-5 | 2,858 (1.4) | 777 (2.7) | 422 (1.3) | 64 (1.2) | 1,073 (1.0) | 522 (1.7) | <.001 | <.001 | <.001 | 0.160 | <.001 |
| 6-19 | 12,466 (6.1) | 2,329 (7.9) | 1,432 (4.5) | 205 (3.9) | 6,458 (6.1) | 2,042 (6.8) | <.001 | <.001 | <.001 | <.001 | <.001 |
| 20-44 | 78,329 (38.6) | 13,700 (46.7) | 12,112 (38.4) | 2,320 (43.7) | 35,917 (33.7) | 14,280 (47.3) | <.001 | <.001 | <.001 | <.001 | <.001 |
| 45-64 | 67,404 (33.2) | 9,466 (32.3) | 11,528 (36.5) | 1,864 (35.1) | 35,524 (33.3) | 9,022 (29.9) | <.001 | <.001 | <.001 | 0.008 | <.001 |
| 65-74 | 22,081 (10.9) | 1,947 (6.6) | 3,565 (11.3) | 498 (9.4) | 13,644 (12.8) | 2,427 (8.0) | <.001 | <.001 | <.001 | <.001 | <.001 |
| 75-84 | 12,250 (6.0) | 807 (2.8) | 1,778 (5.6) | 229 (4.3) | 8,289 (7.8) | 1,147 (3.8) | <.001 | <.001 | <.001 | <.001 | <.001 |
| 85+ | 7,520 (3.7) | 300 (1.0) | 710 (2.3) | 131 (2.5) | 5,642 (5.3) | 737 (2.4) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Female sex, n (%) | 112,292 (55.3) | 16,066 (54.8) | 18,826 (59.7) | 2,953 (55.6) | 58,901 (55.3) | 15,546 (51.5) | <.001 | <.001 | <.001 | 0.160 | <.001 |
| Geographic region, n (%) | |||||||||||
| Northeast | 62,715 (30.9) | 9,865 (33.6) | 8,641 (27.4) | 2,300 (43.3) | 31,485 (29.6) | 10,424 (34.5) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Midwest | 77,538 (38.2) | 8,013 (27.3) | 14,061 (44.6) | 1,517 (28.6) | 46,430 (43.6) | 7,517 (24.9) | <.001 | <.001 | 0.002 | <.001 | <.001 |
| South | 36,776 (18.1) | 7,071 (24.1) | 6,268 (19.9) | 615 (11.6) | 18,685 (17.5) | 4,137 (13.7) | <.001 | <.001 | <.001 | <.001 | <.001 |
| West | 18,458 (9.1) | 3,421 (11.7) | 1,284 (4.1) | 770 (14.5) | 6,743 (6.3) | 6,240 (20.7) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Other | 7,421 (3.7) | 956 (3.3) | 1,293 (4.1) | 109 (2.1) | 3,204 (3.0) | 1,859 (6.2) | <.001 | 0.026 | <.001 | <.001 | <.001 |
| Index month | |||||||||||
| February | 715 (0.4) | 70 (0.2) | 111 (0.4) | 14 (0.3) | 456 (0.4) | 64 (0.2) | <.001 | <.001 | 0.063 | 0.071 | <.001 |
| March | 13,488 (6.7) | 1,613 (5.5) | 3,211 (10.2) | 470 (8.9) | 6,201 (5.8) | 1,993 (6.6) | <.001 | 0.037 | <.001 | <.001 | <.001 |
| April | 38,116 (18.8) | 5,806 (19.8) | 8,420 (26.7) | 1,247 (23.5) | 16,893 (15.9) | 5,750 (19.1) | <.001 | <.001 | <.001 | <.001 | <.001 |
| May | 30,012 (14.8) | 5,183 (17.7) | 4,884 (15.5) | 867 (16.3) | 13,998 (13.1) | 5,080 (16.8) | <.001 | <.001 | <.001 | <.001 | <.001 |
| June | 28,388 (14.0) | 5,270 (18.0) | 3,765 (11.9) | 928 (17.5) | 13,822 (13.0) | 4,603 (15.3) | <.001 | <.001 | <.001 | <.001 | <.001 |
| July | 47,515 (23.4) | 6,718 (22.9) | 6,798 (21.6) | 990 (18.6) | 25,677 (24.1) | 7,332 (24.3) | <.001 | <.001 | <.001 | <.001 | 0.479 |
| August | 32,361 (16.0) | 3,650 (12.5) | 3,340 (10.6) | 574 (10.8) | 20,782 (19.5) | 4,015 (13.3) | <.001 | <.001 | <.001 | <.001 | <.001 |
| September | 12,313 (6.1) | 1,016 (3.5) | 1,018 (3.2) | 221 (4.2) | 8,718 (8.2) | 1,340 (4.4) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Insurance type, n (%) | |||||||||||
| Commercial | 99,636 (49.1) | 12,377 (42.2) | 14,747 (46.8) | 2,820 (53.1) | 57,217 (53.7) | 12,475 (41.3) | <.001 | <.001 | <.001 | 0.389 | <.001 |
| Medicaid | 18,393 (9.1) | 3,915 (13.4) | 4,495 (14.3) | 639 (12.0) | 5,737 (5.4) | 3,607 (12.0) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Medicare | 24,770 (12.2) | 1,439 (4.9) | 4,043 (12.8) | 452 (8.5) | 16,498 (15.5) | 2,338 (7.8) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Multiplee | 15,579 (7.7) | 2,007 (6.8) | 2,968 (9.4) | 282 (5.3) | 8,964 (8.4) | 1,358 (4.5) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Uninsured | 4,380 (2.2) | 1,965 (6.7) | 728 (2.3) | 55 (1.0) | 952 (0.9) | 680 (2.3) | <.001 | <.001 | <.001 | 0.285 | <.001 |
| Missing/unknown | 40,150 (19.8) | 7,623 (26.0) | 4,566 (14.5) | 1,063 (20.0) | 17,179 (16.1) | 9,719 (32.2) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Index BMI category, n (%) | |||||||||||
| <18.5 kg/m2 | 4,348 (3.2) | 681 (3.7) | 682 (2.9) | 143 (4.0) | 2,348 (3.0) | 494 (3.6) | <.001 | 0.149 | <.001 | 0.001 | <.001 |
| 18.5 to <25 kg/m2 | 31,552 (23.0) | 3,282 (17.8) | 3,911 (16.4) | 1,480 (41.1) | 19,453 (25.2) | 3,426 (24.9) | <.001 | <.001 | <.001 | <.001 | 0.562 |
| 25 to <30 kg/m2 | 39,741 (29.0) | 5,624 (30.5) | 5,925 (24.8) | 1,261 (35.0) | 22,445 (29.0) | 4,486 (32.6) | <.001 | <.001 | <.001 | <.001 | <.001 |
| ≥30 kg/m2 | 61,396 (44.8) | 8,832 (48.0) | 13,386 (56.0) | 717 (19.9) | 33,115 (42.8) | 5,346 (38.9) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Baseline Charlson comorbidity score, mean (SD) | 0.51 (1.27) | 0.35 (1.03) | 0.61 (1.41) | 0.37 (1.05) | 0.62 (1.38) | 0.23 (0.86) | <.001 | <.001 | 0.586 | <.001 | <.001 |
| Baseline Charlson comorbidity score category, n (%) | |||||||||||
| 0 | 159,734 (78.7) | 24,640 (84.0) | 23,691 (75.1) | 4,423 (83.3) | 79,912 (75.0) | 27,068 (89.7) | <.001 | <.001 | 0.730 | <.001 | <.001 |
| 1-2 | 29,210 (14.4) | 3,500 (11.9) | 5,169 (16.4) | 661 (12.5) | 17,622 (16.5) | 2,258 (7.5) | <.001 | <.001 | 0.517 | <.001 | <.001 |
| 3-4 | 8,780 (4.3) | 750 (2.6) | 1,653 (5.2) | 147 (2.8) | 5,666 (5.3) | 564 (1.9) | <.001 | <.001 | 0.587 | <.001 | <.001 |
| 5+ | 5,184 (2.6) | 436 (1.5) | 1,034 (3.3) | 80 (1.5) | 3,347 (3.1) | 287 (1.0) | <.001 | <.001 | 0.225 | <.001 | <.001 |
| Baseline comorbidity, n (%) | |||||||||||
| ≥1 comorbidity | 77,549 (38.2) | 9,060 (30.9) | 14,254 (45.2) | 1,683 (31.7) | 46,802 (43.9) | 5,750 (19.1) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Hypertension | 45,406 (22.4) | 4,349 (14.8) | 9,647 (30.6) | 931 (17.5) | 27,568 (25.9) | 2,911 (9.7) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Hyperlipidemia | 38,585 (19.0) | 3,694 (12.6) | 5,909 (18.7) | 976 (18.4) | 25,469 (23.9) | 2,537 (8.4) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Obesity | 27,682 (13.6) | 3,797 (13.0) | 5,846 (18.5) | 312 (5.9) | 15,925 (15.0) | 1,802 (6.0) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Diabetes | 22,590 (11.1) | 3,161 (10.8) | 5,088 (16.1) | 599 (11.3) | 11,965 (11.2) | 1,777 (5.9) | <.001 | 0.030 | <.001 | 0.913 | <.001 |
| CKD | 17,415 (8.6) | 2,014 (6.9) | 3,841 (12.2) | 364 (6.9) | 9,998 (9.4) | 1,198 (4.0) | <.001 | <.001 | <.001 | <.001 | <.001 |
| IHD | 12,156 (6.0) | 878 (3.0) | 1,825 (5.8) | 218 (4.1) | 8,484 (8.0) | 751 (2.5) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Asthma | 12,404 (6.1) | 1,745 (6.0) | 2,602 (8.3) | 223 (4.2) | 6,913 (6.5) | 921 (3.1) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Obstructive sleep apnea | 9,972 (4.9) | 908 (3.1) | 1,906 (6.0) | 129 (2.4) | 6,537 (6.1) | 492 (1.6) | <.001 | <.001 | 0.542 | <.001 | <.001 |
| Heart failure | 7,237 (3.6) | 477 (1.6) | 1,587 (5.0) | 79 (1.5) | 4,691 (4.4) | 403 (1.3) | <.001 | <.001 | <.001 | <.001 | <.001 |
| COPD | 7,324 (3.6) | 366 (1.3) | 1,183 (3.8) | 78 (1.5) | 5,378 (5.1) | 319 (1.1) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Atrial fibrillation | 6,481 (3.2) | 288 (1.0) | 770 (2.4) | 78 (1.5) | 5,064 (4.8) | 281 (0.9) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Inflammatory autoimmune disease | 3,901 (1.9) | 378 (1.3) | 666 (2.1) | 79 (1.5) | 2,511 (2.4) | 267 (0.9) | <.001 | <.001 | 0.011 | <.001 | <.001 |
| Stroke | 2,329 (1.2) | 247 (0.8) | 479 (1.5) | 29 (0.6) | 1,455 (1.4) | 119 (0.4) | <.001 | <.001 | 0.043 | <.001 | <.001 |
| Inflammatory bowel disease | 1,061 (0.5) | 62 (0.2) | 106 (0.3) | 16 (0.3) | 831 (0.8) | 46 (0.2) | <.001 | <.001 | <.001 | <.001 | <.001 |
| HIV | 639 (0.3) | 125 (0.4) | 242 (0.8) | 3 (0.1) | 211 (0.2) | 58 (0.2) | <.001 | <.001 | <.001 | 0.021 | 0.840 |
| Baseline medication use, n (%) | |||||||||||
| Statin | 25,118 (12.4) | 2,483 (8.5) | 4,326 (13.7) | 647 (12.2) | 16,115 (15.1) | 1,547 (5.1) | <.001 | <.001 | <.001 | <.001 | <.001 |
| ACE inhibitor | 13,938 (6.9) | 1,606 (5.5) | 2,639 (8.4) | 258 (4.9) | 8,569 (8.0) | 866 (2.9) | <.001 | <.001 | 0.065 | <.001 | <.001 |
| ARB | 10,518 (5.2) | 923 (3.2) | 2,193 (7.0) | 292 (5.5) | 6,399 (6.0) | 711 (2.4) | <.001 | <.001 | <.001 | 0.128 | <.001 |
| Autoimmune and rheumatic treatments | 1,372 (0.7) | 125 (0.4) | 223 (0.7) | 32 (0.6) | 882 (0.8) | 110 (0.4) | <.001 | <.001 | 0.034 | 0.075 | <.001 |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; Hisp., Hispanic; IHD, ischemic heart disease.
Among the 2,328,387 patients with a COVID-19–related healthcare encounter/test, the proportion with confirmed COVID-19 was 10.1% in the total population and varied significantly by race/ethnicity: Hispanic, 20.2%; Black, 15.2%; Asian, 11.9%; White, 7.8%; Other/unknown, 12.2% (global p<.001).
Ninety-nine percent of patients in the Other/unknown category had unknown race; the remaining 1% comprised those identified as Native Hawaiian/Pacific Islander or American Indian/Alaska Native.
Analysis of variance was used for continuous measures; Pearson chi-square test was used for binary measures.
Two-sample t-test was used for continuous measures; Pearson chi-square test was used for binary measures.
Includes commercial and Medicaid; commercial and Medicare; Medicare and Medicaid; and commercial, Medicare, and Medicaid.
Patients in racial/ethnic minority groups who had confirmed COVID-19 were significantly younger than White patients (mean [SD] age range 41·2 [18·2] to 47·3 [18·9] years vs 49·6 [21·0] years, p < 0·001 for all pairwise comparisons of racial/ethnic minority groups vs White patients) (Table 1). Racial/ethnic differences in comorbidity burden as assessed by Charlson comorbidity scores were generally too small to be clinically meaningful; however, Black patients had a higher burden of hypertension, obesity, diabetes, and kidney disease than White patients (p < 0·001 for all) (Table 1).
3.2. Study outcomes
3.2.1. Index encounter site
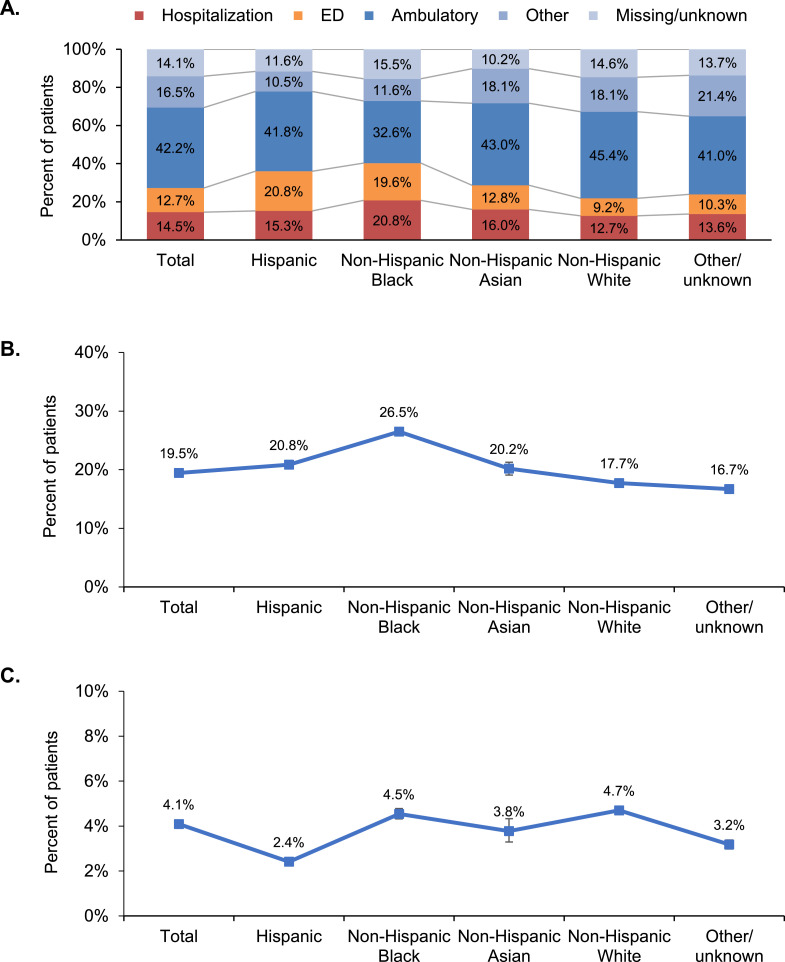
Overall, the largest proportion of index encounters for COVID-19–related care occurred in an ambulatory setting (42·2%); the remainder occurred in the hospital (14·5%), other setting (16·5%), ED (12·7%), or unknown setting (14·1%) (Fig. 1, Panel A). The proportion of patients whose index encounter was an ambulatory visit differed across race/ethnicity groups (global p < 0·001) and was highest among White patients (45·4%) and lowest among Black patients (32·6%) (Fig. 1, Panel A). In contrast, a greater proportion of Hispanic and Black patients than White patients had an ED visit as their index encounter (20·8% and 19·6%, respectively, vs 9·2% for White patients; p < 0·001 for both comparisons). Notably, over one-fifth of Black patients were hospitalized at their index encounter (20·8%, compared with 12·7% of White patients; p < 0·001) (Fig. 1, Panel A). After adjusting for demographics and baseline clinical characteristics, patients from racial/ethnic minority groups were more likely to have a hospitalization as their index encounter; Black patients were 81% more likely than White patients to be hospitalized on initial presentation (odds ratio [OR] [95% CI] 1·810 [1·743–1·880], p < 0·001), while Hispanic patients and Asian patients were 69% and 50% more likely to be hospitalized, respectively, than White patients (OR [95% CI] 1·690 [1·620–1·763] and 1·503 [1·381–1·636], respectively; p < 0·001 for both) (Table 2, Model B).
Fig. 1.
Evidence of racial and ethnic disparities among patients with confirmed COVID-19. In each panel, p < 0.001 for difference across racial/ethnic groups. ED, emergency department.
A, Distribution of index encounter site. Percentages may not sum to 100.0% because of rounding.
B, Follow-up all-cause hospitalization. Error bars represent 95% CIs.
C, Follow-up all-cause mortality. Error bars represent 95% CIs.
Table 2.
Multivariable Logistic Regression Models for Hospitalization and Mortality Among Patients With Confirmed COVID-19
| Models and independent variables | Model Aa |
Model Bb |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Hospitalization on date of index encounter (n=202,908) | ||||
| Hispanic | 1.711 (1.642-1.784) | <.001 | 1.690 (1.620-1.763) | <.001 |
| Non-Hispanic Black | 1.905 (1.836-1.977) | <.001 | 1.810 (1.743-1.880) | <.001 |
| Non-Hispanic Asian | 1.440 (1.325-1.565) | <.001 | 1.503 (1.381-1.636) | <.001 |
| Non-Hispanic White | Ref. | – | Ref. | – |
| Other/unknown | 1.343 (1.287-1.400) | <.001 | 1.379 (1.321-1.441) | <.001 |
| Hospitalization during follow-up (n=202,908) | ||||
| Hispanic | 1.671 (1.611-1.733) | <.001 | 1.700 (1.638-1.764) | <.001 |
| Non-Hispanic Black | 1.673 (1.618-1.729) | <.001 | 1.578 (1.526-1.633) | <.001 |
| Non-Hispanic Asian | 1.272 (1.179-1.371) | <.001 | 1.391 (1.288-1.501) | <.001 |
| Non-Hispanic White | Ref. | – | Ref. | – |
| Other/unknown | 1.152 (1.109-1.197) | <.001 | 1.283 (1.234-1.334) | <.001 |
| Death during follow-up among all patients (n=202,908) | ||||
| Hispanic | 1.026 (0.940-1.121) | 0.565 | 1.041 (0.952-1.138) | 0.375 |
| Non-Hispanic Black | 1.152 (1.077-1.233) | <.001 | 1.110 (1.036-1.189) | 0.003 |
| Non-Hispanic Asian | 1.096 (0.935-1.285) | 0.256 | 1.187 (1.011-1.395) | 0.036 |
| Non-Hispanic White | Ref. | – | Ref. | – |
| Other/unknown | 1.020 (0.943-1.103) | 0.623 | 1.040 (0.960-1.128) | 0.337 |
| Death during follow-up among patients hospitalized during follow-up (n=39,459) | ||||
| Hispanic | 0.965 (0.869-1.073) | 0.514 | 0.977 (0.879-1.087) | 0.673 |
| Non-Hispanic Black | 0.887 (0.816-0.965) | 0.005 | 0.881 (0.809-0.959) | 0.003 |
| Non-Hispanic Asian | 1.177 (0.979-1.416) | 0.083 | 1.205 (1.000-1.452) | 0.050 |
| Non-Hispanic White | Ref. | – | Ref. | – |
| Other/unknown | 1.034 (0.934-1.144) | 0.523 | 1.048 (0.945-1.162) | 0.379 |
Ref., reference.
Adjusted for age group, sex, geographic region, insurance type, and index month. See Supplementary Tables 2-5 for full models.
Adjusted for age group, sex, geographic region, insurance type, index month, index body mass index, baseline Charlson comorbidity score category, baseline comorbidities, and baseline medication use. See Supplementary Tables 2-5 for full models.
3.2.2. Follow-up hospitalization
Overall, 19·5% of patients were hospitalized during follow-up, including those admitted on the index date (Fig. 1, Panel B). The likelihood of hospitalization varied by race/ethnicity group (global p-value < 0·001), with Black patients having the highest proportion of follow-up hospitalizations (26·5%) (Fig. 1, Panel B). After adjusting for demographics and baseline clinical characteristics, Black patients were 58% more likely than White patients to be hospitalized during follow-up (OR [95% CI] 1·578 [1·526–1·633], p <0·001) (Table 2, Model B); patients in other racial/ethnic minority groups were also significantly more likely than White patients to be hospitalized during follow-up (Table 2, Model B; p <0·001 for all). Older age was the strongest predictor of follow-up hospitalization, with patients aged ≥ 85 years having 5-fold higher likelihood of hospitalization than those aged 0–44 (OR [95% CI] 5·052 [4·733–5·392]) (Supplementary Table 3, Model B).
3.2.3. Follow-up death
The unadjusted proportion of deaths during follow-up differed across race/ethnicity groups, with higher mortality observed among White (4·7%) and Black (4·5%) patients and lower mortality observed among Hispanic patients (2·4%) (Figure, Panel C; global p-value < 0·001). After adjusting for demographic differences, Black patients were 15% more likely than White patients to die during the follow-up period (OR [95% CI] 1·152 [1·077–1·233], p <0·001), while differences for other racial/ethnic minority groups relative to White patients were not statistically significant (Table 2, Model A; p > 0·05 for all). However, after further adjustment for baseline clinical characteristics, the likelihood of follow-up death relative to White patients was 11% for Black patients and 19% for Asian patients (OR [95% CI] 1·110 [1·036–1·189], p = 0·003 and 1·187 [1·011–1·395], p = 0·036, respectively) (Table 2, Model B). Older age was the strongest predictor of follow-up death. Odds ratios (95% CI) relative to patients aged 0–44 years ranged from 7·344 (6·443–8·371) for ages 45–64 to 94·685 (82·006–109·324) for ages 85 and above (p < 0·001 for all) (Supplementary Table 4, Model B).
Among the subset of patients hospitalized during follow-up, Black patients were significantly less likely to die than White patients after adjusting for demographics and baseline clinical characteristics (OR [95% CI] 0·881 [0·809–0·959], p = 0·003), but Asian patients had a 21% higher adjusted likelihood of death than White patients (OR [95% CI] 1·205 [1·000–1·452], p = 0·050) (Table 2, Model B). Differences in mortality between Hispanic patients and White patients were not statistically significant (OR [95% CI] 0·977 [0·879–1·087], p = 0·673) (Table 2, Model B).
4. Discussion
The results of this retrospective study of 202,908 US patients with confirmed COVID-19 provide additional context to a large body of evidence that members of racial and ethnic minority groups—and the Black population, in particular—bear a disproportionate burden of morbidity and mortality due to the COVID-19 pandemic in the US [[13], [14], [15], [16], [17],[19], [20], [21], [22], [23],25,26,31]. Although older age was the strongest risk factor for hospitalization and death, patients from racial/ethnic minority groups were more likely than White patients to be hospitalized or visit the ED upon initial presentation and to require hospitalization during the course of illness, despite being significantly younger. This finding is consistent with prior research [13,19,[21], [22], [23],25,26] and likely driven by differential distribution of disease severity by race/ethnicity among confirmed COVID-19 cases, due in part to underrepresentation of patients with asymptomatic disease from racial/ethnic minority groups in the study population.
Our observation that members of racial/ethnic minority groups required a higher intensity of health care at initial presentation with COVID-19 supports this hypothesis and is congruent with previous studies indicating Black patients hospitalized for COVID-19 are more likely to present with worse vital signs and more severe disease on chest x-ray, which is associated with poorer clinical outcomes [18,32]. These findings suggest that patients from racial/ethnic minority groups may receive testing and/or treatment for COVID-19 later in the disease process than White patients, potentially missing the opportunity for early interventions that could help improve outcomes. Delayed access to COVID-19–related resources may be due in part to differential access to healthcare resources, as patients from racial/ethnic minority groups are more likely than White patients to be uninsured [33] and to reside in communities lacking in physicians and other health services [5,[34], [35], [36]]. However, testing and treatment delays may also be attributable to individual reluctance to pursue medical care. Mistrust of the healthcare system resulting from familiarity with historical mistreatment of marginalized groups in medical research, as well as personal experiences of discriminatory treatment and/or substandard medical care in healthcare settings, are important barriers to receiving healthcare among racial/ethnic minority populations [6,37]. Such mistrust has been associated not only with healthcare underutilization but also with increased use of the ED as usual site of care, consistent with our results [6,7,38,39]. Additional factors likely contributing to delayed presentation among patients with COVID-19 from racial/ethnic minority groups include essential worker status; [11,12] lack of paid time off to seek medical care; [40] suboptimal understanding of COVID-19 symptoms warranting medical attention; [41] and the myth that Black people are immune to the coronavirus, which arose from early reports of low COVID-19 infection rates in Africa [42].
It is also possible that COVID-19 disease progression among some racial/ethnic minority groups is exacerbated by a generally higher comorbidity burden [13,18,31]. We observed that greater proportions of Black patients vs White patients with COVID-19 had diabetes, obesity, hypertension, and kidney disease, which have been associated with poorer outcomes; [43,44] moreover, adjusting for baseline clinical status in addition to demographics reduced Black patients’ risk of death during follow-up from approximately 15% to 11% relative to White patients, suggesting that a portion of mortality risk among Black patients with COVID-19 may be attributable to comorbidities.
Notably, adjustment for comorbidities increased the risk of follow-up death for Asian patients relative to White patients, and among those who were hospitalized, Asian patients remained more likely to die than White patients. These findings suggest that for people of Asian descent, the disparity in mortality outcomes may be mediated by other mechanisms. Socioeconomic and cultural factors such as disproportionate representation in high-contact essential occupations, higher prevalence of multigenerational households, and limited access to critical healthcare information among individuals whose primary language is not English may all play a role; [45] however, data regarding these factors are not readily available in the EHR database and could not be analyzed in the present study.
Disparate access to healthcare resources among racial/ethnic minority groups—and the resulting disproportionate prevalence of chronic conditions associated with increased COVID-19 severity—are multifaceted problems driven by longstanding systemic inequities, and their dismantling will require solutions of similar scale and complexity. Our findings highlight increased access to healthcare as a critical element of such solutions. Although we found that Black, Hispanic, and Asian patients diagnosed with COVID-19 were more likely than White patients to require hospitalization, outcomes of hospitalization for some racial/ethnic minority groups were similar to those of White patients. Our results echo those from other large retrospective analyses of patients with COVID-19 in which adjusted in-hospital mortality was similar or lower for Black patients relative to White patients [18,21,31,46]. Together, these findings suggest that equitable in-hospital care may help to equalize racial/ethnic disparity in COVID-19 mortality rates, [47] underscoring the need to promote earlier entry of racial/ethnic minorities into the healthcare system for COVID-19–related preventive services and treatment. Strategies to be considered include expanding access to COVID-19 vaccination, testing, and screening in marginalized communities; conducting culturally appropriate outreach to members of underrepresented racial/ethnic groups to emphasize the importance of vaccination as well as early testing and prompt medical attention for suspected COVID-19; and ensuring that cost or fear of income loss are not barriers to receipt of care [48]. Such endeavors are imperative given the availability of highly effective preventive vaccines and antiviral treatments that are most effective when administered early in the course of infection.
Including over 202,000 patients from healthcare systems across the US, this is the largest and most diverse analysis of racial and ethnic disparities in COVID-19 to date; however, it also has certain limitations. First, because the EHR data are sourced from hospital systems and integrated delivery networks (IDNs), the study sample is heavily weighted towards hospitalized patients. Those receiving ambulatory care outside of the hospital systems and IDNs in our database, testing positive at public testing sites, or not seeking formal medical care are not represented; therefore, our results are limited to patients seeking COVID-19–related care, may undercount deaths occurring outside of the hospital, and are more representative of patients with higher disease severity. Second, race/ethnicity information was not available for a substantial number of patients identified with confirmed COVID-19, which also may have affected the generalizability of the results; furthermore, outcomes among individuals identified as American Indian/Alaska Native or Native Hawaiian/other Pacific Islander or reporting multiple race categories could not be analyzed because these groups were included in the Other/unknown category to maintain patient privacy, given their small sample sizes (< 0·2% of patients altogether). Third, information such as patient occupation, education level, economic status, detailed geographic location, and urban/rural environment were not available. These factors are known to contribute to disparities among racial/ethnic minority groups and may also affect COVID-19 infection risk, testing availability, and treatment access, [49,50] but could not be analyzed in this study. Fourth, as we did not require a minimum history of clinical activity in the EHR, a subset of patients may have been new to the system because of COVID-19 triaging (eg, patients who were transferred from out-of-system hospitals in other communities due to capacity issues); in addition, not every pertinent detail of a patient's health status is reflected in EHR, especially for patients who do not typically seek care within the contributing healthcare systems. These factors may have resulted in incomplete capture of comorbid conditions and other clinical details. Furthermore, information on smoking status, which has been shown to affect COVID-19 outcomes, [51] was not captured. Finally, although the definition used to identify patients’ first COVID-19–related healthcare encounter was relatively expansive, it is possible that some relevant encounters were not attributed to COVID-19 and therefore were missed. Conversely, some patients with incorrect COVID-19 diagnoses may have been inadvertently included. These scenarios may have been more likely early in the study period, when there was still considerable uncertainty around COVID-19 symptoms and a lack of formal ICD-10 codes to identify COVID-19–related care; however, 84.9% of diagnosed patients also had a positive PCR or antigen test, somewhat mitigating this concern.
In this nationwide sample of patients with confirmed COVID-19, patients from underrepresented racial and ethnic groups were more likely than White patients to receive care in a hospital setting on initial presentation and to require hospitalization during their disease course; these differences could not be explained by differences in demographics and comorbidity burden. Black and Asian patients were also more likely to die than White patients; however, mortality risk was lower among Black patients who were hospitalized, highlighting the importance of equitable access to health care in the community setting. While further analyses of population-level data on COVID-19 care and clinical outcomes disaggregated by race and ethnicity are still needed, our findings confirm, on a larger scale, observations from studies with smaller patient samples. Now that the COVID-19 pandemic has helped bring longstanding racial and ethnic health disparities into sharp focus, it is incumbent on the healthcare community to not only facilitate timely access to COVID-19 testing and vaccination for underserved racial and ethnic minority populations, but also work toward identifying meaningful and sustainable policies to address the underlying systemic inequities that are driving these disparities.
Funding
No funding was received for this work.
Data sharing statement
Data collected for this study will not be made available to others, as the Optum EHR Database contains proprietary elements owned by Optum.
Authors' contributions
Ami Buikema, Paul Buzinec, Brian Solow, and U. Michael Currie conceptualized the study. Misti Paudel, Katherine Andrade, Jonathan Johnson, Benjamin Chastek, Harish Raja, Feng Cao, Erin Hulbert, Dibyajyoti Mazumder, Sumit Jhamb, and Stephanie Korrer collected and analyzed the data. Yvette Edmonds drafted the manuscript. All authors interpreted the results, critically reviewed and edited the manuscript, and approved the final version for submission.
Declaration of Competing Interest
Ami Buikema, Paul Buzinec, Katherine Andrade, Jonathan Johnson, Yvette Edmonds, Sumit Jhamb, Benjamin Chastek, Harish Raja, Feng Cao, Erin Hulbert, Stephanie Korrer, Dibyajyoti Mazumder, Jerry Seare, and Brian Solow report that they are employees of Optum, which provided access to the data used in this research. Misti Paudel reports that she was an employee of Optum at the time this study was conducted. U. Michael Currie reports that he is an employee of UnitedHealth Group.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101075.
Appendix. Supplementary materials
References
- 1.Congressional Research Service. COVID-19: US economic effects. 2020; Available at: https://crsreports.congress.gov/product/pdf/IN/IN11388. Accessed 16 October 2020.
- 2.American Hospital Association. Hospitals and health systems face unprecedented financial pressures due to COVID-19. 2020; Available at: https://www.aha.org/guidesreports/2020-05-05-hospitals-and-health-systems-face-unprecedented-financial-pressures-due. Accessed 16 October 2020.
- 3.US Centers for Disease Control and Prevention. CDC COVID data tracker. 2020; Available at: https://covid.cdc.gov/covid-data-tracker/. Accessed 20 July 2021.
- 4.APM Research Lab. The color of coronavirus: COVID-19 deaths by race and ethnicity in the US. 2020; Available at: https://www.apmresearchlab.org/covid/deaths-by-race. Accessed 15 September 2020.
- 5.Richardson L.D., Norris M. Access to health and health care: how race and ethnicity matter. Mt Sinai J Med. 2010;77(2):166–177. doi: 10.1002/msj.20174. [DOI] [PubMed] [Google Scholar]
- 6.Webb Hooper M., Mitchell C., Marshall V.J. Understanding multilevel factors related to urban community trust in healthcare and research. Int J Environ Res Public Health. 2019;16(18):3280. doi: 10.3390/ijerph16183280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musa D., Schulz R., Harris R., Silverman M., Thomas S.B. Trust in the health care system and the use of preventive health services by older black and white adults. Am J Public Health. 2009;99(7):1293–1299. doi: 10.2105/AJPH.2007.123927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajilore O., Thames A.D. The fire this time: the stress of racism, inflammation and COVID-19. Brain Behav Immun. 2020;88:66–67. doi: 10.1016/j.bbi.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb Hooper M., Napoles A.M., Perez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frydman G.H., Boyer E.W., Nazarian R.M., Van Cott E.M., Piazza G. Coagulation status and venous thromboembolism risk in African Americans: a potential risk factor in COVID-19. Clin Appl Thromb Hemost. 2020;26(1076029620943671) doi: 10.1177/1076029620943671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts J.D., Dickinson K.L., Koebele E. Clinicians, cooks, and cashiers: examining health equity and the COVID-19 risks to essential workers. Toxicol Ind Health. 2020;36(9):689–702. doi: 10.1177/0748233720970439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkins D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. Am J Ind Med. 2020;63(9):817–820. doi: 10.1002/ajim.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulson M., Geary A., Annesi C. National disparities in COVID-19 outcomes between Black and White Americans. J Natl Med Assoc. 2021;113(2):125–132. doi: 10.1016/j.jnma.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anyane-Yeboa A., Sato T., Sakuraba A. Racial disparities in COVID-19 deaths reveal harsh truths about structural inequality in America. J Intern Med. 2020;288(4):479–480. doi: 10.1111/joim.13117. [DOI] [PubMed] [Google Scholar]
- 15.Moore J.T., Ricaldi J.N., Rose C.E. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5-18, 2020 - 22 states, February-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(33):1122–1126. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross C.P., Essien U.R., Pasha S., Gross J.R., Wang S.Y., Nunez-Smith M. Racial and ethnic disparities in population-level Covid-19 mortality. J Gen Intern Med. 2020;35(10):3097–3099. doi: 10.1007/s11606-020-06081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes L., Jr., Enwere M., Williams J. Black-White risk differentials in COVID-19 (SARS-COV2) transmission, mortality and case fatality in the United States: translational epidemiologic perspective and challenges. Int J Environ Res Public Health. 2020;17(12):4322. doi: 10.3390/ijerph17124322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yehia B.R., Winegar A., Fogel R. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golestaneh L., Neugarten J., Fisher M. The association of race and COVID-19 mortality. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vahidy F.S., Nicolas J.C., Meeks J.R. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-039849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karaca-Mandic P., Georgiou A., Sen S. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states. JAMA Intern Med. 2021;181(1):131–134. doi: 10.1001/jamainternmed.2020.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azar K.M.J., Shen Z., Romanelli R.J. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff. 2020;39(7):1253–1262. doi: 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- 24.McCarty T.R., Hathorn K.E., Redd W.D. How do presenting symptoms and outcomes differ by race/ethnicity among hospitalized patients with coronavirus disease 2019 infection? Experience in Massachusetts. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1245. ciaa1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nau C., Bruxvoort K., Navarro R.A. COVID-19 Inequities across multiple racial and ethnic groups: results from an integrated health care organization. Ann Intern Med. 2021;M20-8283 doi: 10.7326/M20-8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escobar G.J., Adams A.S., Liu V.X. Racial disparities in COVID-19 testing and outcomes : retrospective cohort study in an integrated health system. Ann Intern Med. 2021;174(6):786–793. doi: 10.7326/M20-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Optum, Inc. Optum EHR data. 2021; Available at: https://www.optum.com/business/solutions/life-sciences/real-world-data/ehr-data.html. Accessed 02 June 2021.
- 28.US Department of Health & Human Services. The HIPAA privacy rule. 2020; Available at: https://www.hhs.gov/hipaa/for-professionals/privacy/index.html. Accessed 28 June 2021.
- 29.US Centers for Disease Control and Prevention. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. 2020; Available at: https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf. Accessed 14 September 2020.
- 30.Quan H., Li B., Couris C.M. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 31.Ogedegbe G., Ravenell J., Adhikari S. Assessment of racial/ethnic disparities in hospitalization and mortality in patients With COVID-19 in New York City. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph N.P., Reid N.J., Som A. Racial/ethnic disparities in disease severity on admission chest radiographs among patients admitted with confirmed COVID-19: a retrospective cohort study. Radiology. 2020;297(3):E303–E312. doi: 10.1148/radiol.2020202602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolbert, J., Orgera, K. Key facts about the uninsured population. 2020; Available at: https://www.kff.org/uninsured/issue-brief/key-facts-about-the-uninsured-population/. Accessed 28 June 2021.
- 34.Gaskin D.J., Dinwiddie G.Y., Chan K.S., McCleary R.R. Residential segregation and the availability of primary care physicians. Health Serv Res. 2012;47(6):2353–2376. doi: 10.1111/j.1475-6773.2012.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guadamuz J.S., Wilder J.R., Mouslim M.C., Zenk S.N., Alexander G.C., Qato D.M. Fewer pharmacies in black and Hispanic/Latino neighborhoods compared with white or diverse neighborhoods, 2007-15. Health Aff. 2021;40(5):802–811. doi: 10.1377/hlthaff.2020.01699. [DOI] [PubMed] [Google Scholar]
- 36.Chan K.S., Gaskin D.J., McCleary R.R., Thorpe R.J. Availability of health care provider offices and facilities in minority and integrated communities in the US. J Health Care Poor Underserved. 2019;30(3):986–1000. doi: 10.1353/hpu.2019.0069. [DOI] [PubMed] [Google Scholar]
- 37.Smith A.C., Woerner J., Perera R., Haeny A.M., Cox J.M. An investigation of associations between race, ethnicity, and past experiences of discrimination with medical mistrust and COVID-19 protective strategies. J Racial Ethn Health Disparities. 2021;11:1–13. doi: 10.1007/s40615-021-01080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnett M.J., Thorpe R.J., Jr., Gaskin D.J., Bowie J.V., LaVeist T.A. Race, medical mistrust, and segregation in primary care as usual source of care: findings from the exploring health disparities in integrated communities study. J Urban Health. 2016;93(3):456–467. doi: 10.1007/s11524-016-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell W., Richmond J., Mohottige D., Yen I., Joslyn A., Corbie-Smith G. Medical mistrust, racism, and delays in preventive health screening among African-American men. Behav Med. 2019;45(2):102–117. doi: 10.1080/08964289.2019.1585327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez J., Islam T., Beller J., Fiori K., Correa R., Correa D.J. Expanding paid sick leave as a public health tool in the COVID-19 pandemic. J Occup Environ Med. 2020;62(10):e598–e599. doi: 10.1097/JOM.0000000000001998. [DOI] [PubMed] [Google Scholar]
- 41.Alsan M., Stantcheva S., Yang D., Cutler D. Disparities in coronavirus 2019 reported incidence, knowledge, and behavior among US adults. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins-Dexter, B. Canaries in the coal mine: COVID-19 misinformation and Black communitites. 2020; Available at: https://shorensteincenter.org/canaries-in-the-coal-mine/. Accessed 25 May 2021.
- 43.Gao F., Zheng K.I., Wang X.B. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43(7):e72–e74. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- 44.Mantovani A., Byrne C.D., Zheng M.H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30(8):1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang, E., Huang, S., Kwok, A., Lung, H., Park, M., Yueh, E. COVID-19 and advancing Asian American recovery. 2020; Available at: https://www.mckinsey.com/industries/public-and-social-sector/our-insights/covid-19-and-advancing-asian-american-recovery. Accessed 24 November 2020.
- 46.Asch D.A., Islam M.N., Sheils N.E. Patient and hospital factors associated with differences in mortality rates among black and white US medicare beneficiaries hospitalized with COVID-19 infection. JAMA Netw Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.12842. [DOI] [PubMed] [Google Scholar]
- 47.Boulware L.E. Race disparities in the COVID-19 pandemic-Solutions lie in policy, not biology. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.18696. [DOI] [PubMed] [Google Scholar]
- 48.UnitedHealth Group. Advancing health equity to improve outcomes. 2021; Available at: https://www.unitedhealthgroup.com/viewer.html?file=/content/dam/UHG/PDF/About/Advancing-Health-Equity-to-Improve-Outcomes.pdf. Accessed 09 March 2021.
- 49.Braveman P., Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. 2014;129(Suppl 2):19–31. doi: 10.1177/00333549141291S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan T.Q., Kullar R., Swartz T.H., Mathew T.A., Piggott D.A., Berthaud V. Location matters: geographic disparities and impact of coronavirus disease 2019. J Infect Dis. 2020;222(12):1951–1954. doi: 10.1093/infdis/jiaa583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy R.K., Charles W.N., Sklavounos A., Dutt A., Seed P.T., Khajuria A. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol. 2021;93(2):1045–1056. doi: 10.1002/jmv.26389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.