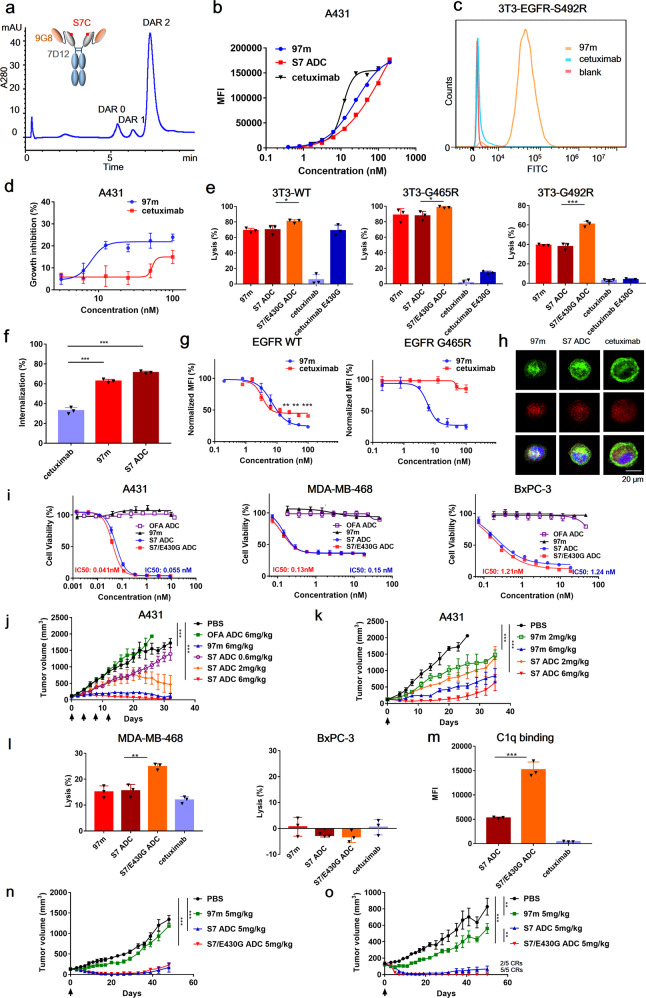
Fig. 1.
Multivalent anti-EGFR nanobody drug conjugate. a Schematic diagram of 97m and Hydrophobic interaction chromatography (HIC) analysis of S7 ADC. b Flow cytometry analysis of concentration-dependent binding of cetuximab, 97m and S7 ADC with EGFR-positive A431 cells. c Binding ability of biparatopic nanobody 97m and cetuximab with EGFR-S492R NIH-3T3 cells. d The inhibition effect of 97m and cetuximab on A431 tumor cell proliferation. e CDC activity of biparatopic nanobody and its conjugates on EGFR-wt, EGFR-G465R, and EGFR-S492R NIH-3T3 cells. f Internalization of cetuximab, 97m and S7 ADC in A431 cells. g Quantification of internalized and degraded EGFR through flow cytometry. h Intracellular trafficking and lysosomal localization of cetuximab, 97m and S7 ADC in A431 cells at 37 °C for 1.5 h antibodies (green), lysosomes (red), nucleus (blue). i In vitro cytotoxicity of S7 ADC and S7/E430G ADC on A431, MDA-MB-468, and BxPC-3 tumor cells. j In vivo antitumor activities of multi-dose 97m and S7 ADC in A431 xenograft models. k In vivo antitumor activities of single-dose 97m and S7 ADC in A431 xenograft models. l CDC activity of 97m and its conjugates on MDA-MB-468 and BxPC-3 cells. m Flow cytometry analysis of C1q deposition on A431 cells in the presence of S7 ADC, S7/E430G ADC, or cetuximab. n In vivo antitumor activities of single-dose S7 ADC and S7/E430G ADC in BxPC-3 xenograft models. o In vivo antitumor activities of single-dose S7 ADC and S7/E430G ADC in MDA-MB-468 xenograft models