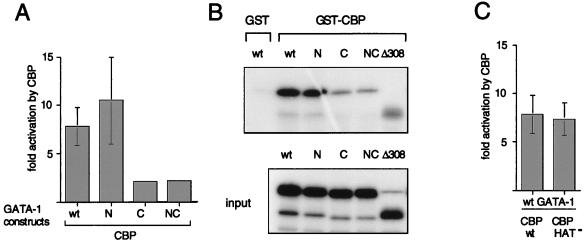
FIG. 6.
(A) Stimulation of GATA-1 activity by CBP depends on an intact C-motif. In transient-transfection experiments with NIH 3T3 cells, a GATA-binding site-containing artificial promoter-reporter, M1αGH (1 μg), was cotransfected with 2 μg of wild-type (wt) or mutant (K-to-A substitutions) GATA-1 in the presence or absence of 7 μg of pCMV5-CBP. Fold activation represents the ratio of activity obtained in the presence and absence of CBP. (B) The C-motif of GATA-1 participates in CBP binding. GST pull-down assays were performed with wild-type and mutant in vitro-translated [35S]methionine-labeled GATA-1. GST-CBP, which represents the GATA-1-binding domain (aa 1805 to 1891), served as an affinity reagent. (C) The acetyltransferase activity of CBP is dispensable for GATA-1 activation in transient-transfection assays. Experiments were performed as in panel A. CBP-HAT− represents an acetyltransferase-defective mutant of CBP bearing a double point mutation in the HAT domain.