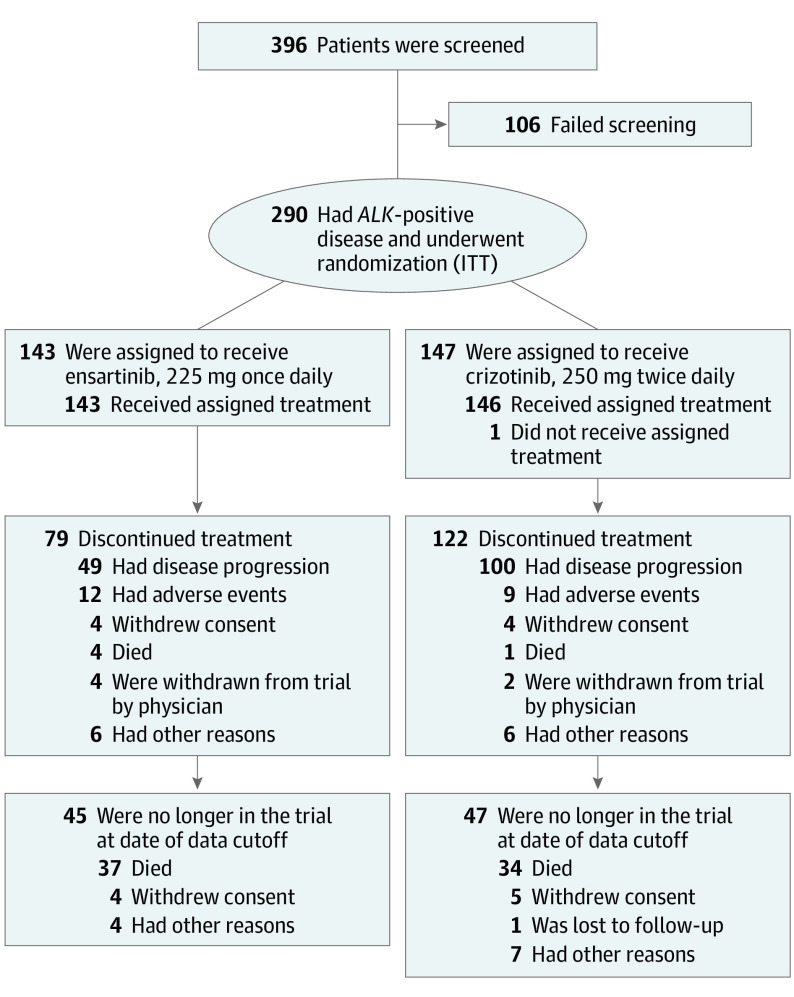
Figure 1. Screening, Enrollment, Randomization, and Follow-up.
Data reported as of the cutoff for the first interim analysis (July 1, 2020) are shown. In the ensartinib group, 49 patients had documented disease progression according to the Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1. In the crizotinib group, 100 patients had documented disease progression according to RECIST, version 1.1. ALK indicates anaplastic lymphoma kinase gene; ITT, intent to treat.