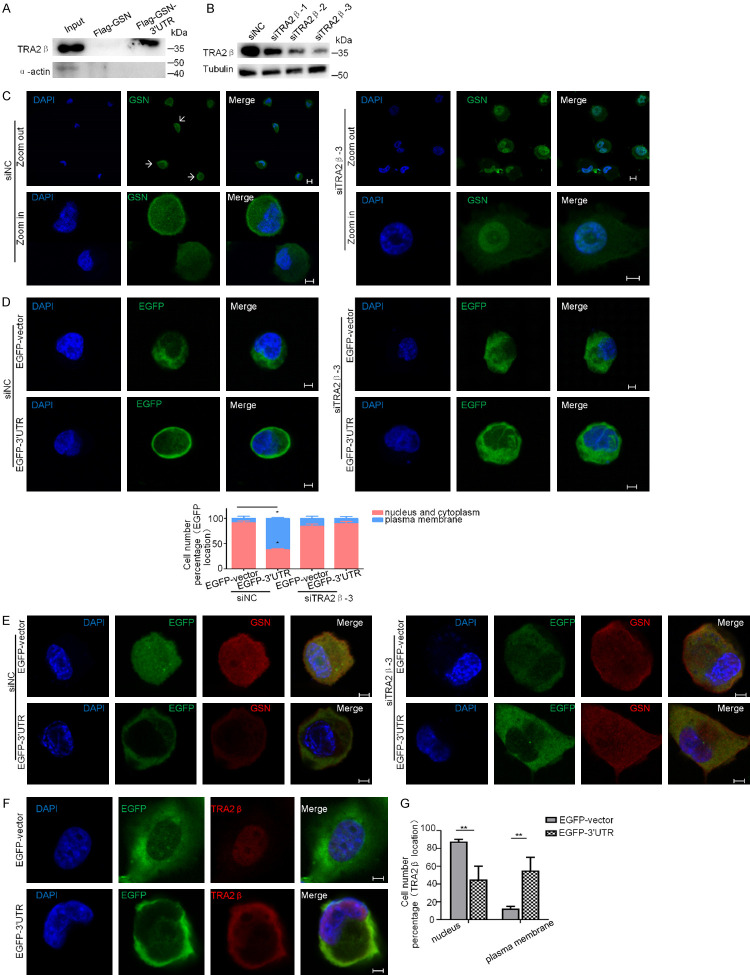
Figure 6.
Tra2β bound to GSN 3’UTR and was important for the localized expression of GSN. (A) RNA pull-down assay. H1299 cell lysate was incubated with in vitro synthesized Flag-GSN transcript or Flag-GSN-3’UTR transcript as described in Materials and methods. Western blot analysis was performed to detect Tra2β protein bound specifically to GSN 3’UTR. α-actin was used as a negative control. (B) Verification of Tra2β knockdown in H1299 cells using three siRNAs by Western blotting. β-tubulin was used for internal control. (C) H1299 cells were transfected with siTra2β-3 or siNC, and then immunostained with antibodies against GSN (green). Nuclear DNA was stained with DAPI (blue). Arrows: endogenous GSN proteins at the proximity of the plasma membrane. (D, E) H1299 cells were subsequently transfected with siNC or siTra2β-3 for 24 h, and with EGFP-3’UTR or EGFP-vector for another 24 h, then subjected to immunofluorescence confocal microscopy for the subcellular localization of EGFP (D) or endogenous GSN protein (E). (F, G) Immunofluorescence analyses the subcellular localization of EGFP and endogenous Tra2β protein in H1299 cells transfected with EGFP-vector and EGFP-3’UTR plasmids. The cell number with each indicated expression pattern was quantified from three independent experiments (> 15 cells each, n=3). Scale bars: 10 μm.