Figure 3.
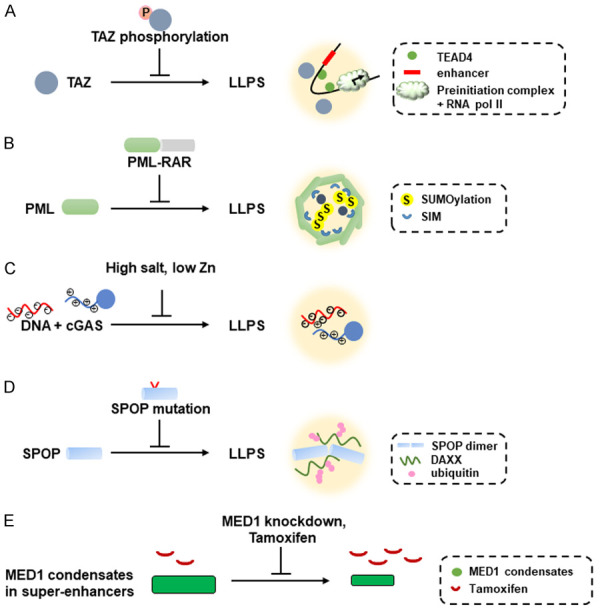
Disruption of LLPS through different mechanisms causes different human diseases as well as how chemotherapeutic drugs inhibit activation of oncogenes through shrinkage of MED1 condensates. The specific diseases are described in the text. A. Phosphorylation of the coiled-coil domain of TAZ inhibits the TAZ-mediated LLPS that involves TAZ, TEAD4, BRD4, and CDK9. B. PML-RAR chimeric proteins disrupt the formation of PML NBs that is mediated by SUMOylated PML proteins containing SIMs (SUMO-interacting motifs). C. Negatively-charged DNA interacts with positively-charged N-terminal domain of cGAS to form LLPS. High salt or low Zn concentration disfavor the LLPS formation between DNA and cGAS. D. Formation of LLPS between oligomerized SPOP and DAXX could be disrupted by mutated SPOP that is observed in prostate cancer and breast cancer. DAXX ubiquitination is also inhibited by SPOP mutations in cancer. E. Overexpression of MED1 in Tamoxifen-resistant breast cancer cells causes formation of large MED1 condensates and leads to dilution of Tamoxifen concentration in the condensates. Knockdown of MED1 or Tamoxifen treatment decreases the size of MED1 condensates and increases the concentration of Tamoxifen inside the MED1 condensates, leading to chemosensitivity to Tamoxifen.
