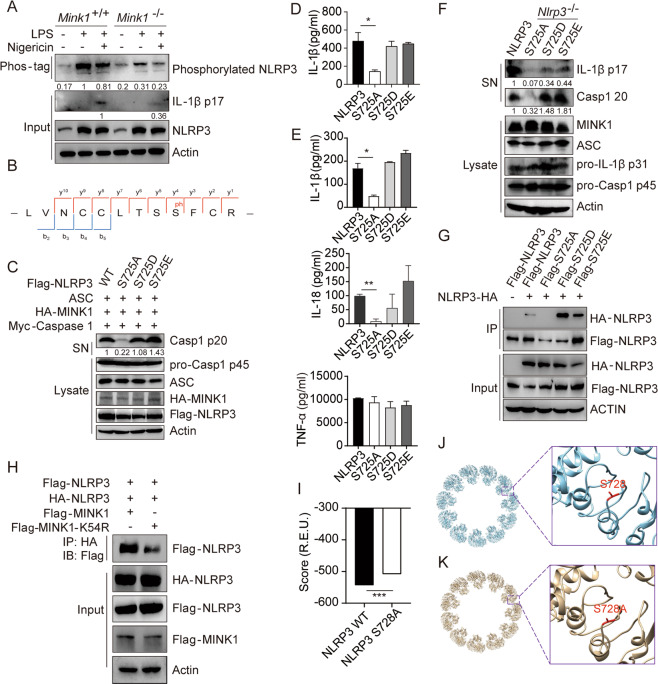
Fig. 4.
MINK1-mediated NLRP3 phosphorylation at Ser725 is critical for inflammasome activation. A Phos-tagTM SDS–PAGE was used to detect phosphorylated NLRP3 in BMDMs primed with LPS and stimulated with nigericin. B IP was performed after overexpressing MINK1 and NLRP3 in HEK293T cells, followed by phosphorylation mass spectrometry to show the phosphorylated residue. C, D Reconstruction of NLRP3 activation in HEK293T cells, supernatants (SN) and cell extracts (lysate) was determined using immunoblotting (C). IL-1β release was determined using ELISA (D). ELISA analyses of IL-1β, IL-18, and TNF-α in culture supernatants (E) or immunoblot analysis of p17 and p20 (F) from Nlrp3−/− BMDMs transduced with mouse WT or mutant NLRP3-expressing lentivirus and primed with LPS and stimulated with ATP. G IP and immunoblot analysis of the aggregation of NLRP3 after overexpressing HA-NLRP3 with different Flag-NLRP3 in HEK293T cells. H IP and immunoblot analysis of the aggregation of NLRP3 after overexpressing Flag-MINK1, Flag-MINK1-K54R, and different tagged NLRP3 in HEK293T cells. I–K The structural model of WT NLRP3-NEK is shown in blue, and serine 728 is shown in red. The inset shows the detailed conformation (J), and the S728A mutant is shown in K. The inset shows the detailed conformation. The score (in the unit of R.E.U.) reflecting the structural stability was calculated by Rosetta for WT NLRP3-NEK and the S728A mutant (I). *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA was used for D and E, two-tailed unpaired Student’s t test was used for I. The ELISA and western blot results are representative of three independent experiments