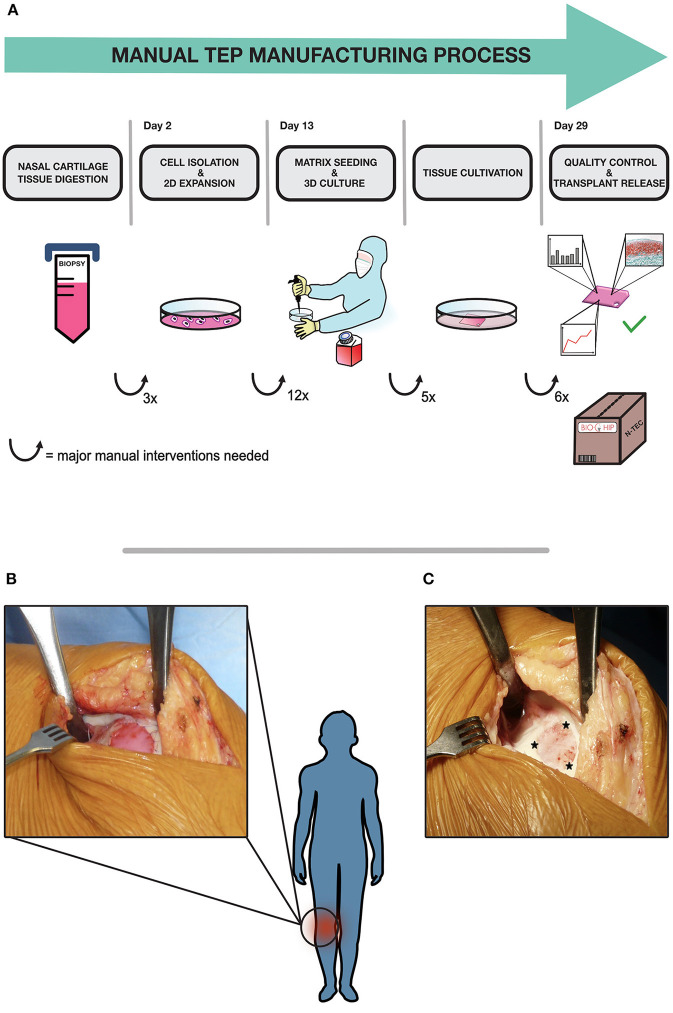
Figure 1.
BIO-CHIP manual manufacturing process and surgical procedure. The whole process is graphically depicted in (A). An autologous cartilage biopsy is taken via outpatient surgery in a clinical site from the patients' nasal septum according to SOP. The biopsy is shipped to the manufacturing site, where the tissue is digested and the cartilage cells are isolated and expanded in-vitro. In the next step, cells are manually seeded on a collagen membrane in a certified cleanroom facility. Various parameters are monitored continuously throughout tissue cultivation. After 2 weeks of static tissue culture, final quality testing is conducted. The amount of extracellular matrix proteins is evaluated using histological grading by modified Bern score, also cell viability and transplant stability are assessed. When all defined release criteria are met, the N-TEC is packed and sent back to the clinic to be implanted into defect site in a surgical procedure (B). The patch is secured by surrounding absorbable sutures during the surgical procedure. The initial focal cartilage defect in the knee is depicted in (C), asterisks indicate the defect site where the N-TEC is inserted.