Abstract
Background and Aims
Phosphorus (P) and nitrogen (N) are essential nutrients that frequently limit primary productivity in terrestrial ecosystems. Efficient use of these nutrients is important for plants growing in nutrient-poor environments. Plants generally reduce foliar P concentration in response to low soil P availability. We aimed to assess ecophysiological mechanisms and adaptive strategies for efficient use of P in Banksia attenuata (Proteaceae), naturally occurring on deep sand, and B. sessilis, occurring on shallow sand over laterite or limestone, by comparing the allocation of P among foliar P fractions.
Methods
We carried out pot experiments with slow-growing B. attenuata, which resprouts after fire, and faster growing opportunistic B. sessilis, which is killed by fire, on substrates with different P availability using a randomized complete block design. We measured leaf P and N concentrations, photosynthesis, leaf mass per area, relative growth rate and P allocated to major biochemical fractions in B. attenuata and B. sessilis.
Key Results
The two species had similarly low foliar total P concentrations, but distinct patterns of P allocation to P-containing fractions. The foliar total N concentration of B. sessilis was greater than that of B. attenuata on all substrates. The foliar total P and N concentrations in both species decreased with decreasing P availability. The relative growth rate of both species was positively correlated with concentrations of both foliar nucleic acid P and total N, but there was no correlation with other P fractions. Faster growing B. sessilis allocated more P to nucleic acids than B. attenuata did, but other fractions were similar.
Conclusions
The nutrient allocation patterns in faster growing opportunistic B. sessilis and slower growing B. attenuata revealed different strategies in response to soil P availability which matched their contrasting growth strategy.
Keywords: Leaf phosphorus fractions, phosphate, phospholipid, phosphorus allocation, photosynthetic P use efficiency, nucleic acid, relative growth rate
INTRODUCTION
Understanding strategies of nutrient allocation and their underlying mechanisms in plants adapted to phosphorus- (P) impoverished soils is an important topic in plant physiological ecology (Lambers et al., 2006; Veneklaas et al., 2012). Phosphorus-impoverished soils limit the growth and yield of crops, pastures and forests throughout the world (Conroy et al., 1990; Herbert and Fownes, 1995; Seneweera and Conroy, 1997; Fujita et al., 2003; Thomas et al., 2006). Moreover, a recent meta-analysis showed that P limitation of above-ground plant production is pervasive in natural terrestrial ecosystems (Hou et al., 2020). Low soil P availability is widespread in Australia (Viscarra Rossel and Bui, 2016; Kooyman et al., 2017), and plants generally respond to low soil P availability by having a low foliar P concentration (Epstein and Bloom, 2005).
Foliar P concentration is the sum of the concentrations of several major P fractions in leaf cells including inorganic P (Pi) and various P-containing organic compounds (i.e. nucleic acids, phospholipids and small phosphate esters; Veneklaas et al., 2012). The allocation of P to foliar fractions is likely to be related to life history strategy, because these fractions are functionally related to growth, reproduction and stress tolerance (Hidaka and Kitayama, 2011). Shifting P allocation patterns in leaves is an important mechanism for plants to acclimate to low soil P availability (Hidaka and Kitayama, 2011; Yan et al., 2019). If strong P limitation occurs, plants shift the allocation of P among foliar P fractions, and this might increase their fitness under the prevailing conditions (Hidaka and Kitayama, 2011).
Chapin and Kedrowski (1983) found no evidence in four Alaskan tree species for adaptations to nutrient stress through major changes in biochemical use of nitrogen (N) and P by investigating foliar P fractions; however, they found important changes in allocation of N and P to different leaf fractions, during the growing season (Chapin and Kedrowski, 1983). Hidaka and Kitayama (2009) found that plants growing on P-impoverished tropical soils increased both leaf mass per area (LMA) and photosynthetic P use efficiency (PPUE) compared with plants on P-richer soil. These authors suggested that a greater proportion of cellular P may be allocated to metabolic P, rather than to structural P to maintain high PPUE. Yan et al. (2019) investigated foliar P fractions of three species along a 2 million year chronosequence with a strong gradient of available P in south-western Australia, and found that their P allocation pattern was associated with their distribution along the chronosequence, concluding that the differences are likely to be adaptive. How plants allocate P among foliar P fractions and exhibit adaptive strategies to use P efficiently in two species in the same genus with contrasting life history strategies in extremely P-impoverished ecosystems with a Mediterranean climate remains unclear.
Comparison of the relationship between growth rate and P investment showed that species with fast growth rates exhibit low N:P ratios (Elser and Hamilton, 2007). This pattern has been explained by the growth rate hypothesis (GRH), which proposes that fast growth rates require greater allocation of resources (mainly P) to P-rich ribosomal RNA (rRNA) to meet the protein synthesis demands needed to support the rapid growth rates (Elser et al., 1996; Sterner and Elser, 2002; Elser and Hamilton, 2007; Reef et al., 2010; Hidaka and Kitayama, 2011). Nucleic acids have an N:P stoichiometry of 4:1 (Reef et al., 2010), and are a major fraction of organic P, with RNA by far the largest proportion (Geider and La Roche, 2002). Within the RNA pool, rRNA is the largest P fraction.
Tree species in ancient landscapes have experienced long-term low soil P status; thus, they probably possess adaptations to P limitation. Non-mycorrhizal Proteaceae are particularly successful on the most P-impoverished soils in south-western Australia (Pate et al., 2001; Lambers et al., 2013; Hayes et al., 2014). These species with specialized cluster roots effectively mine soil P by releasing carboxylates to replace P sorbed to soil particles (Shane and Lambers, 2005) and exhibit relatively fast rates of area-based photosynthesis, despite having extremely low leaf P concentrations (Denton et al., 2007; Lambers et al., 2012; Sulpice et al., 2014), while leaves of P-starved crop plants tend to have slow rates of photosynthesis per unit leaf area (Brooks et al., 1988; Rao et al., 1989; Fredeen et al., 1990). Consequently, some of these Proteaceae exhibit a very high PPUE (Denton et al., 2007; Lambers et al., 2010; Sulpice et al., 2014). This high PPUE in Proteaceae from severely P-impoverished habitats is brought about mainly by low foliar rRNA concentrations (Sulpice et al., 2014) and extensive replacement of phospholipids by galactolipids and sulfolipids during leaf development (Lambers et al., 2012).
The slow-growing resprouter Banksia attenuata and the faster growing seeder B. sessilis (Pate et al., 1991) both inhabit low-P soils and produce compound cluster roots (Shane and Lambers, 2005), but have different life histories (Shi et al., 2020); the soil total N concentrations in their natural habitats is 0.24–0.27 g kg–1 (Hayes et al., 2014). Banksia sessilis is a short-lived obligate seeder that occurs on shallow sand over laterite or limestone (Pate and Bell, 1999; Hayes et al., 2019) and allocates more biomass to cluster roots than B. attenuata, which invests more in deep roots (Shi et al., 2020). This strategy enhances P mobilization from laterite or limestone by releasing more carboxylates and/or exuding these at a faster rate than B. attenuata (Shi et al., 2020). In contrast to B. sessilis, B. attenuata is restricted to deep sand (FloraBase, http://florabase.dpaw.wa.gov.au/), grows slowly and is long lived (Pate et al., 1990; Knox and Clarke, 2005; Bowen and Pate, 2017). McArthur and Wilson (1967) coined the terms r strategy and K strategy to describe selection for rapid population growth in uncrowded populations and selection for competitive ability in crowded populations, respectively. Over time, the meaning of these terms has broadened (Parry, 1981) and, according to the broader concept, B. sessilis is an r strategist, while B. attenuata is a K strategist. We do not know the physiological pattern of allocating P among foliar P fractions that allows species to exhibit a particular life history strategy and efficient use of P in contrasting low-P environments. Therefore, we aimed to compare P allocation patterns in these two Banksia species with contrasting life history. Thus, we measured leaf P and N concentrations, LMA, and concentrations and proportions of P in foliar P-containing fractions in B. attenuata and B. sessilis grown with different substrate P availability.
We hypothesized that: (1) with decreasing soil P availability, the foliar total P concentrations of both B. attenuata and B. sessilis would decrease; and (2) B. sessilis, which exhibits a more opportunistic r strategy than B. attenuata, would have a higher foliar NTotal:PTotal ratio and invest more P in nucleic acids than B. attenuata when grown on the same substrate.
MATERIALS AND METHODS
Sowing method
Seeds of Banksia attenuata R.Br. and B. sessilis (Knight) A.R. Mast & K.R. Thiele (purchased from Nindethana Seed Company, King River, Western Australia) were sown on filter paper. One seedling was transferred into each experimental pot on 22 May 2016. According to the supplier, the seeds of B. sessilis were collected from a coastal population, growing over limestone near Jurien Bay, Western Australia (30°18′S, 115°3′E). The provenance of the B. attenuata seed was unknown.
Experimental design
A pot experiment was carried out in a glasshouse at the University of Western Australia, Perth, Australia (31°59′S, 115°53′E) using a randomized complete block design. Glasshouse temperature fluctuated between 13 and 33 °C over a whole year, and transmission of radiation into the glasshouse was 60 % of natural light. The experiment was designed to explore why B. sessilis is able to grow across a wider range of P-impoverished soil types and maintain a greater relative growth rate (RGR) than B. attenuata by comparing the use and allocation of P among foliar P fractions in the two species. Three soil treatments were imposed, based on washed river sand: sand only, sand + laterite (SLAT) and sand + limestone (SLIM). The substrate total P availability was sand > SLAT > SLIM (Supplementary data Fig. S1; Shi et al., 2020). The pots (100 mm inner diameter × 400 mm tall PVC cylinders) were lined with plastic bags. For each soil treatment, 3.0 kg of substrate was added to the pots. For the SLAT and SLIM treatment, a 100 mm layer of laterite or limestone gravel, respectively, was added 50 mm below the soil surface, and other layers were filled with sand. There were ten replicates for each species in each treatment. Field capacity of soils in each treatment was calculated as [(wet mass – dry mass)/dry mass] × 100 %. The pots were watered to a constant weight of 80 % of field capacity three times a week. A 20 mL aliquot of basal liquid nutrient solution lacking P and containing (per kg of soil) 217.5 mg of KNO3; 74 mg of CaCl2; 140 mg of K2SO4; 80 mg of MgSO4·7H2O; 28.9 mg of MnSO4·H2O; 10 mg of ZnSO4·7H2O; 5 mg of CuSO4·5H2O; 0.7 mg of H3BO3; 0.5 mg of CoSO4·7H2O; 0.4 mg of Na2MoO4·2H2O; and 20 mg of FeNaEDTA was applied to each pot once every second week.
Photosynthesis measurement
Prior to the final harvest, the net photosynthetic rate (Pn) of attached leaves was measured between 10.00 and 11.00 h on 7 and 9 March 2017 using a red/blue LED light source (LI-6400, LI-COR Inc., Lincoln, NE, USA). The plants were watered on the day before the photosynthesis measurement. One mature leaf of each plant was measured at a photosynthetic photon flux density of 1500 μmol m–2 s–1 and a CO2 concentration of 400 μmol mol–1. The leaves used for photosynthesis measurements were sampled, and the projected leaf area measured at 200 dpi (Epson 1680, Long Beach, CA, USA) and calculated (ImageJ 1.4, NIH, Bethesda, MD, USA). Leaves were then dried at 70 °C for 72 h to measure dry mass (DM).
Harvest
After 50 weeks of growing in pots, a total of 20 fully expanded leaves with no visible damage or discolouration were harvested from each plant. The leaves were immediately scanned at 200 dpi to calculate leaf area (LA1), then submerged in liquid nitrogen and stored at –80 °C. Frozen leaves were freeze-dried for 7 d (VirTis Benchtop ‘K’, New York, USA) and DM was determined (DM1). The remaining leaves on each plant were harvested and scanned at 200 dpi to calculate the remaining leaf area (LA2). Total LA = LA1 + LA2. The remaining leaves, stem and roots were separated and dried at 70 °C for 72 h. The DM was determined for the remaining leaves (DM2) and for stems plus roots (DM3). Total leaf DM = DM1 + DM2. Total plant dry mass M2 = DM1 + DM2 + DM3. The LMA was calculated as total leaf DM/total LA. Seed weight (W1) was measured using four lots of ten (B. attenuata) or 30 (B. sessilis) seeds that were dried (70 °C, 48 h) and weighed before calculating the average seed weight. The RGR was calculated as (lnM2 – lnW1)/(T2 – T1), where T1 and T2 are the dates of sowing and harvesting, respectively, expressed in weeks.
Leaf nutrient analyses
Freeze-dried leaves were ground to a fine powder (Geno/Grinder 2010, Spex SamplePrep, Metuchen, NJ, USA). A 50 mg sample was used to determine inorganic P (Pi; Yan et al., 2019).
The P allocated to the nucleic acid, lipid, small metabolite (Pi + other metabolites) and residual fractions was determined in a 50 mg portion of powdered leaves using the differential solubility method (Hidaka and Kitayama, 2013), as modified by Yan et al. (2019). Metabolite P is defined here as small metabolite P – Pi.
Phosphorus concentrations in extracts and residues from the above procedures were measured as described by Matusiewicz and Golik (2004) using the molybdenum blue method (Ames, 1966). Total leaf P is the sum of Pi, nucleic acids, lipids, metabolites and the residual fraction. Total leaf P was confirmed by acid digestion of ground leaf material, followed by Pi assay. Total foliar N concentration was determined by combustion of approx. 30 mg of dried leaf sample (Vario Macro Combustion Analyser, Elementar Analysensysteme GmbH, Langenselbold, Germany).
Leaf area-based P concentration was calculated as total leaf P concentration × LMA; PPUE was calculated as the ratio of photosynthesis rate to area-based P concentration; leaf area-based N concentration was calculated as leaf N concentration × LMA; and photosynthetic N use efficiency (PNUE) was calculated as the ratio of photosynthesis rate to area-based N concentration.
Statistics
The differences in means between B. attenuata and B. sessilis on the same substrate were analysed by two-way analysis of variance (ANOVA) with 95 % confidence intervals, while the differences in means within each species across substrate types were analysed by Tukey’s method. The relationships of foliar P fractions to total foliar P concentration, LMA and RGR to nucleic acid P, and foliar N and foliar N to nucleic acid P were determined by linear regression analysis; the correlation coefficients were analysed by Student’s t-test. All statistical analyses were performed using the SPSS Statistics 19.0 (SPSS Inc., Chicago, IL, USA), and graphed with OriginPro 9.5 (OriginLab Corporation, Northampton, MA, USA).
RESULTS
Leaf P and N concentrations
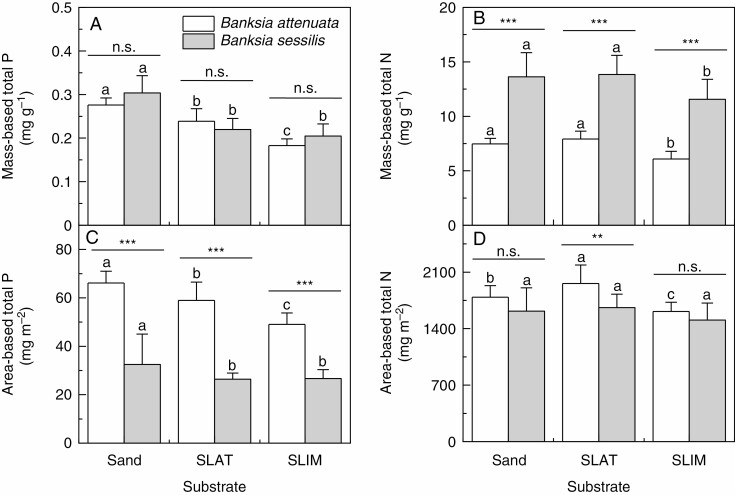
The effect of P availability on P and N relationships in leaves of B. attenuata and B. sessilis was tested by growing plants in sand, SLAT and SLIM. Foliar total P concentrations on a mass basis for both B. attenuata and B. sessilis were highest when grown in sand, and lowest in SLIM (Fig. 1A). However, there were no significant differences between B. attenuata and B. sessilis in foliar total P concentrations for any substrate type (Fig. 1A). Mass-based foliar total P concentrations for B. attenuata differed on all three substrates (P < 0.05): sand > SLAT > SLIM. In contrast to foliar total P concentration, foliar total N concentration on a mass basis for B. sessilis was greater than that for B. attenuata in each substrate type (Fig. 1B). The leaf total N concentrations on a mass basis for both species were the same in sand and SLAT, and higher than when grown in SLIM (P < 0.05).
Fig. 1.
Leaf mass-based total P (A), and N (B) concentrations and area-based P (C) and N (D) concentrations of two Banksia species, Banksia attenuata and B. sessilis, that were grown in different substrates. Values are means ± s.e. (n = 10); the asterisks denote significant differences between the species by t-test. n.s., not significant, **P < 0.01, ***P < 0.001. Different letters indicate significant differences among substrates (P < 0.05). SLAT, sand plus laterite; SLIM, sand plus limestone.
The differences in mass-based foliar total P concentration across substrates for both B. attenuata and B. sessilis were also apparent when P concentrations were expressed on an area basis (P < 0.05; Fig. 1C). However, on an area basis, the foliar P concentration for B. attenuata was higher than that for B. sessilis in all substrates. There were also substrate-dependent differences in area-based foliar total N concentration for B. attenuata, but not for B. sessilis (Fig. 1D). In contrast to higher mass-based foliar N concentrations in B. sessilis than in B. attenuata in all substrates, the area-based foliar N concentration was higher in B. attenuata in SLAT, or was not distinguishable (Fig. 1D).
The concentration ratio of foliar total N to foliar total P (NTotal:PTotal) had the same pattern for the two species across the three substrate types (Table 1). The NTotal:PTotal ratio for both species was lower for plants grown in sand than for those grown in SLAT or SLIM (P < 0.05). However, there was no significant difference for either species when grown in SLAT or SLIM. The NTotal:PTotal ratio in B. sessilis was significantly higher than that in B. attenuata in every substrate.
Table 1.
Mass ratios for total foliar nitrogen (N) to total foliar phosphorus (P) and to P in each foliar P-containing fraction, and relative growth rate (RGR) for two Banksia species, B. attenuata and B. sessilis, grown in different substrates
| Items | Banksia attenuata | Banksia sessilis | ||||
|---|---|---|---|---|---|---|
| Sand | SLAT | SLIM | Sand | SLAT | SLIM | |
| Leaf N/total leaf P | 27.1 ± 1.8b | 33.4 ± 2.5a | 33.1 ± 3.6a | 45.9 ± 8.9b*** | 63.2 ± 7.4a*** | 56.9 ± 7.72a*** |
| Leaf N/Pi | 100 ± 14b | 161 ± 46a | 176 ± 46a | 253 ± 86b*** | 390 ± 67a*** | 356 ± 80a*** |
| Leaf N/lipid P | 159 ± 25a | 168 ± 27a | 158 ± 23a | 294 ± 76b*** | 436 ± 127a*** | 398 ± 95ab*** |
| Leaf N/metabolic P | 119 ± 10b | 168 ± 36a | 177 ± 38a | 189 ± 29b*** | 274 ± 41a*** | 239 ± 54a** |
| Leaf N/nucleic acid P | 105 ± 9a | 114 ± 11a | 108 ± 13a | 136 ± 29b** | 186 ± 28a*** | 159 ± 25ab*** |
| Leaf N/residual P | 416 ± 94a | 447 ± 94a | 375 ± 66a | 692 ± 195a*** | 607 ± 98a** | 772 ± 258a*** |
| RGR (mg g–1 week–1) | 95 ± 2.6a | 93 ± 3.7a | 86 ± 4.6b | 123 ± 4a*** | 115 ± 7.6b*** | 110 ± 6.1b*** |
Values are means ± s.e. (n = 10); asterisks indicate significant differences between the species by t-test. **P < 0.01, ***P < 0.001. Different letters indicate significant differences for the two species among the substrates (P < 0.05). SLAT, sand plus laterite; SLIM, sand plus limestone.
Photosynthetic rates, PPUE and PNUE
Net photosynthetic rates (Pn) for B. attenuata grown in SLIM were lower than for plants grown in sand or SLAT, while there were no substrate-dependent differences for B. sessilis (P > 0.05; Table 2). There were no differences in photosynthesis rate between the two species in any substrate (P > 0.05). Moreover, there were no substrate-dependent differences in PPUE within either species (Table 2). However, the PPUE of B. sessilis was significantly higher than that of B. attenuata in all three substrates (P < 0.05; Table 2). The PNUE for B. attenuata grown in sand was higher than that of plants grown in SLIM (P < 0.05; Table 2), while PNUE for B. sessilis was the same in all substrate types (Table 2). Moreover, there were no significant differences in PNUE between B. attenuata and B. sessilis for any substrate type (Table 2).
Table 2.
Photosynthesis rates, photosynthetic P use efficiency (PPUE), photosynthetic N use efficiency (PNUE) and foliar mass per area (LMA) of two Banksia species grown in different substrates: B. attenuata and B. sessilis
| Items | Banksia attenuata | Banksia sessilis | ||||
|---|---|---|---|---|---|---|
| Sand | SLAT | SLIM | Sand | SLAT | SLIM | |
| Photosynthesis rates (µmol m–2 s–1) | 15.3 ± 3a | 15.4 ± 1.3a | 10.8 ± 1.8b | 13.4 ± 2.2an.s. | 13.2 ± 6.2an.s. | 10.7 ± 2.3an.s. |
| PPUE (µmol g–1 P s–1) | 234 ± 53a | 264 ± 38a | 223 ± 52a | 377 ± 86.4a*** | 506 ± 269a* | 410 ± 116a*** |
| PNUE (µmol g–1 N s–1) | 8.9 ± 2.2a | 7.9 ± 1.0ab | 6.7 ± 1.0b | 8.6 ± 2.4an.s. | 7.9 ± 3.5an.s. | 7.3 ± 1.9an.s. |
| LMA (g m–2) | 240 ± 15b | 248 ± 21b | 267 ± 24a | 119 ± 7b** | 121 ± 9b** | 131 ± 6a** |
Values are means ± s.e. (n = 10); asterisks indicate significant differences between the species by t-test. n.s., not significant, *P < 0.05, **P < 0.01, ***P < 0.001. Different letters indicate significant differences for the species among the substrates (P < 0.05). SLAT, sand plus laterite; SLIM, sand plus limestone
Leaf P fractions
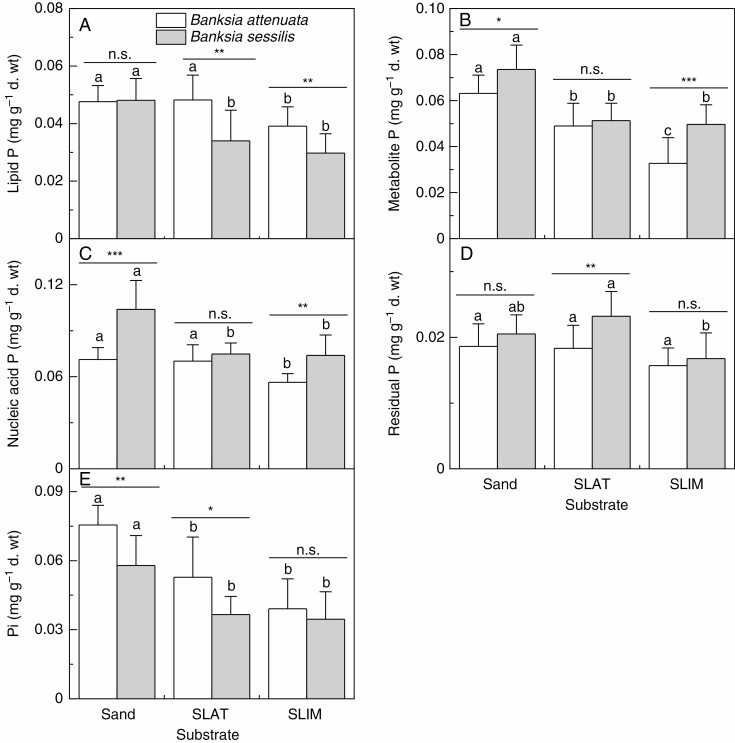
Banksia attenuata and B. sessilis had different patterns of allocating leaf P to lipid, metabolite, nucleic acid and residual fractions in all substrates (Fig. 2). The lipid P concentrations of B. attenuata grown in SLIM were lower than in plants grown in sand and SLAT (Fig. 2A), while the difference in metabolite P concentration in B. attenuata grown in the three substrate types was sand > SLAT > SLIM (Fig. 2B). The nucleic acid P concentrations of B. attenuata grown in SLIM were lower than in plants grown in sand and SLAT (Fig. 2C). The lipid P, metabolite P, nucleic acid P and Pi concentrations of B. sessilis grown in sand were greater than those in plants grown in SLAT and SLIM (Fig. 2A–C, E).
Fig. 2.
The lipid phosphorus (P) (A), metabolite P (B), nucleic acid P (C), residual P (D) and inorganic P (Pi) concentrations (E) in leaves of two Banksia species, B. attenuata and B. sessilis, grown in different substrates. Values are means ± s.e. (n = 10); asterisks indicate significant differences between the two species by t-test. n.s., not significant, *P < 0.05, **P < 0.01, ***P < 0.001. Different letters indicate significant differences for the two species among substrates (P < 0.05). SLAT, sand plus laterite; SLIM, sand plus limestone.
The lipid P concentration of B. attenuata was greater than that of B. sessilis in SLAT and SLIM (Fig. 2A). The metabolite P and nucleic acid P concentrations of B. attenuata were lower than those of B. sessilis in both sand and SLIM, but were the same in SLAT (Fig. 2B, C). The residual P concentration only differed between the species when grown in SLAT, with the concentration in B. attenuata being lower than that in B. sessilis (Fig. 2D). The Pi concentrations of B. attenuata were greater than those of B. sessilis in sand and SLAT, but not in SLIM (Fig. 2E).
The NTotal:PFraction ratios for metabolite P and Pi for B. attenuata were significantly lower in sand than in SLAT or SLIM, while the ratios for the other fractions were indistinguishable among the substrates (Table 1). This result shows that metabolite P and Pi fractions were drivers for the observed difference in leaf NTotal:PTotal ratio for B. attenuata in the three substrates. For B. sessilis, all P factions except residual P contributed to the lower leaf NTotal:PTotal ratio in sand. The ratios of leaf NTotal:PFraction in each P fraction were lower in B. attenuata than in B. sessilis for plants grown in any of the three substrates (Table 2).
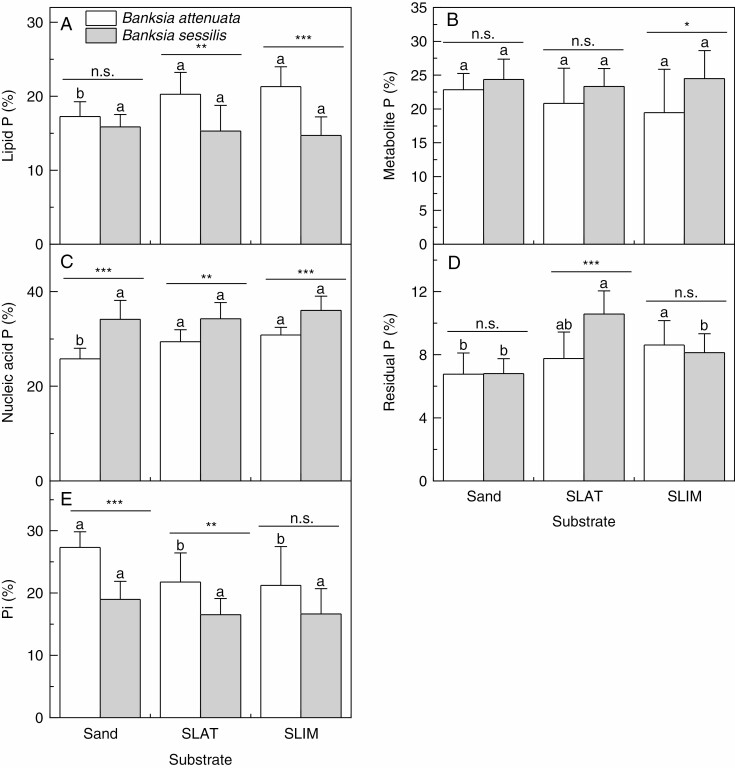
The proportions of foliar P fractions to total foliar P
The proportions of lipid P and nucleic acid P in B. attenuata were significantly lower for plants grown in sand than for those grown in SLAT or SLIM (P < 0.05; Fig. 3A, C). Conversely, the proportion of total P in Pi for B. attenuata was greater for plants grown in sand than for plants grown in SLAT or SLIM (P < 0.05; Fig. 3E). There was no significant difference in the proportions of total P in lipids, metabolites, nucleic acids and Pi for B. sessilis grown in any of the three substrates (Fig. 3A).
Fig. 3.
The proportions of total foliar phosphorus (P) as lipid P (A), metabolite P (B), nucleic acid P (C), residual P (D) and inorganic P (Pi) (E) for two Banksia species, B. attenuata and B. sessilis, grown in different substrates. Values are means ± s.e. (n = 10); asterisks indicate significant differences between the two species by t-test. n.s., not significant, *P < 0.05, **P < 0.01, ***P < 0.001. Different letters indicate significant differences for the two species among substrate types (P < 0.05). SLAT, sand plus laterite; SLIM, sand plus limestone.
The proportion of total P in lipid P in B. attenuata was greater than that of B. sessilis in SLAT and SLIM (P < 0.01; Fig. 3A). Conversely, the proportion of total P in nucleic acid P in B. sessilis was significantly greater than that in B. attenuata in all substrates (Fig. 3C); likewise, the proportion of metabolite P in B. sessilis was greater than that in B. attenuata in SLIM (Fig. 3B). The proportion of residual P was <10 % in all substrates, except for B. sessilis grown in SLAT (Fig. 3D). The proportion of total P in Pi in B. attenuata was greater than that of B. sessilis in sand and SLAT (P < 0.01; Fig. 3E).
The relationships of RGR and foliar nutrient concentrations, foliar P fractions and leaf mass per area
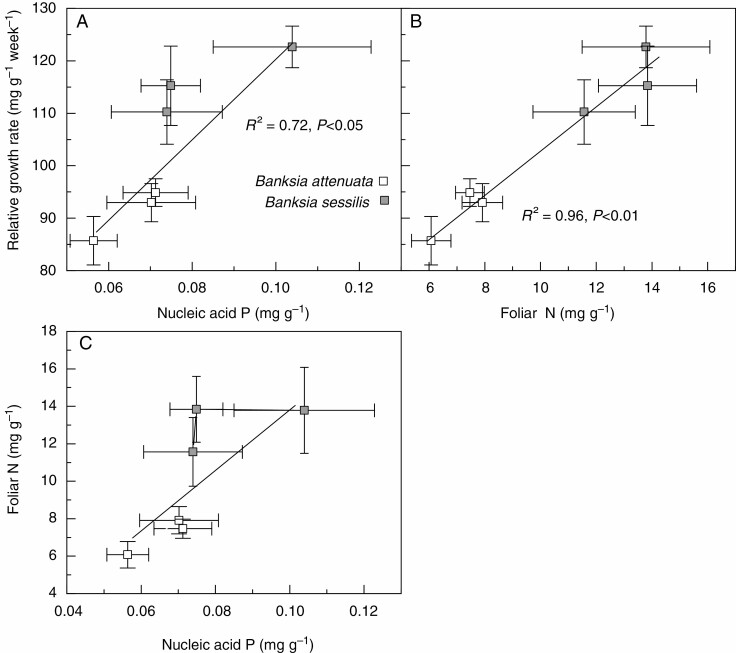
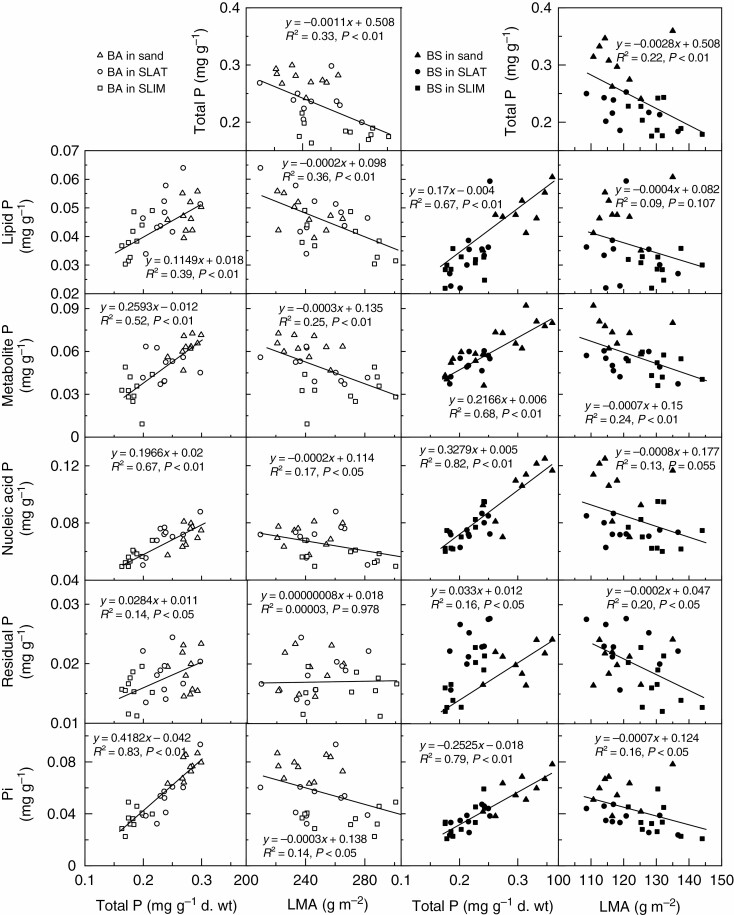
The RGR of B. attenuata was slower than for B. sessilis in all substrates (Table 1). In B. attenuata, the RGR was the same in sand and SLAT, but slower in SLIM, while in B. sessilis, RGR was also fastest in sand, but slower and equal in both SLAT and SLIM. The RGR of B. attenuata and B. sessilis was positively correlated with foliar nucleic acid P concentration (Fig. 4A) and foliar N concentration (Fig. 4B). There was also a positive correlation between foliar N concentration and nucleic acid P concentration for both species (Fig. 4C). No other correlations were detected in any other pairwise comparison of P concentration with N concentration. In both species, the concentrations of P in lipid, metabolite, nucleic acid, residual and Pi fractions were all correlated positively with foliar total P concentration (Fig. 5).
Fig. 4.
Relationships of relative growth rate (RGR) to foliar nucleic acid phosphorus (P), foliar nitrogen (N), and foliar N to nucleic acid P for Banksia attenuata and B. sessilis. Values are means ± s.e. (n = 10). Individual data can be found in Figs 1 and 2, and Table 1.
Fig. 5.
Relationships between foliar phosphorus (P) fractions and total foliar P concentration (left), and leaf mass per area (LMA) (right) for Banksia attenuata and B. sessilis grown in three substrates (n = 30). Each symbol represents an individual plant. BA, B. attenuata; BS, B. sessilis; SLAT, sand plus laterite; SLIM, sand plus limestone.
The LMA varied from 240 to 267 g m–2 in B. attenuata and from 119 to 131 g m–2 in B. sessilis depending on the growth substrate (Fig. 5; Table 2). The concentrations of the P fractions and total P generally decreased with increasing LMA (Fig. 5). This relationship was not significant for lipid P and nucleic acid P in B. sessilis. The only exception to a negative correlation between leaf P fraction and LMA was the lack of a relationship between residual P and LMA in B. attenuata.
Discussion
Foliar total P and N concentrations
Leaf N and P concentration can be expressed on a leaf area (Narea) or leaf mass basis (Nmass; Li et al., 2016). Concentrations on an area basis are useful when comparing rates of photosynthesis expressed on an area basis. However, mass-based values are useful in terms of expenditures and returns per unit investment, and allow broader comparisons with the literature (Wright et al., 2004). Our first hypothesis was that with decreasing soil P availability, the mass-based foliar total P concentrations of both B. attenuata and B. sessilis would decrease, and this was supported (Fig. 1). An important finding was that the area-based foliar P concentrations were higher in B. attenuata than in B. sessilis in all three substrates, but there was no significant difference between B. attenuata and B. sessilis in mass-based foliar P concentration for any substrate type. We found the same patterns of mass-based foliar total P concentration for both B. attenuata and B. sessilis grown in all three substrates; the foliar total P concentrations of both species were greatest in sand, and about 35 % lower in SLIM. The greatest carboxylate-extractable P concentration was in sand, and the lowest was in limestone gravel (Supplementary data Fig. S1).
In contrast to the similar mass-based foliar total P concentrations in the two species in each substrate, the mass-based foliar total N concentration of B. sessilis was almost twice that of B. attenuata grown in the same substrate. Leaf N concentrations in B. attenuata and B. sessilis grown on SLIM were approx. 20 % lower than those in plants grown in sand and SLAT. Our result differs from results on Hakea prostrata (Proteaceae), showing that P availability did not influence leaf N concentration (Prodhan et al., 2016). The foliar total N concentrations of both species were very low compared with those of plants from other environments (Reich et al., 1991), which reflects the low foliar rRNA concentration in B. attenuata (Sulpice et al., 2014) and, presumably, in B. sessilis, based on the similar size of their nucleic acid P pools. In our study, the relatively low foliar N concentrations in B. attenuata and B. sessilis indicate that protein concentrations were very low, which implies a low demand for rRNA and, thus, P.
Whilst the leaf N concentrations in both species were low compared with the global average (Reich et al., 1991; Wright et al., 2004), they were distinctly higher in B. sessilis than in B. attenuata. The higher N concentration correlated with greater allocation of P to the nucleic acid P fraction in B. sessilis. However, rates of photosynthesis and leaf N concentrations expressed on an area basis were similar for the two species, and hence so was the PNUE. Therefore, the ‘extra’ N in B. sessilis on a mass basis was a reflection of a lower investment in sclerenchymatic tissue, as evidenced by its lower LMA. A low ribosome abundance can be expected to decrease the rate of protein synthesis, and hence the protein and N concentrations. Therefore, lower leaf N concentrations in B. attenuata compared with B. sessilis on the three substrates tested is consistent with lower rRNA concentrations and lower rates of protein synthesis. However, since we studied mature non-growing leaves, which do not rapidly change in protein concentration (Kuppusamy et al., 2014), the faster rate of protein synthesis must have been balanced by a faster rate of protein breakdown, and hence protein turnover (Sulpice et al., 2014).
According to the GRH, species with rapid growth rates would have low foliar N:P ratios, because of their high P-rich rRNA concentrations. In our analysis, we found that RGR was strongly correlated with leaf N and nucleic acid P concentrations in both species; this would appear to support the GRH within each species, but we should bear in mind that our results on N and P concentrations refer to fully expanded leaves that had stopped growing. Also, RGR, N concentration and foliar N:P in B. sessilis were significantly greater than those in B. attenuata when grown in the same substrate. This supports our second hypothesis that B. sessilis, which exhibits a more opportunistic growth strategy than B. attenuata (Shi et al., 2020), has a higher foliar NTotal:PTotal ratio than B. attenuata, and invests more P in nucleic acid P to support a higher N concentration. This finding is in line with a study on Pinus species in a glasshouse experiments (Matzek and Vitousek, 2009). The higher leaf N concentration found here and the higher capacity to acquire P (Shi et al., 2020) in B. sessilis than in B. attenuata when grown in the more P-limiting SLIM may explain the different distribution patterns of the two species. A higher capacity to acquire P presumably allows it to colonize and become established on different P-impoverished soils (sand over laterite or over limestone), compared with B. attenuata, which is restricted to deep sand (FloraBase, http://florabase.dpaw.wa.gov.au/).
Foliar traits and P fractions
The different foliar P allocation patterns combined with differences in LMA between the two species reflects differences in their life history strategies and resource requirements. Plants like B. sessilis with an r selection life history typically grow fast (Clarke et al., 2013) and produce seeds before the next catastrophe, i.e. fire or drought (Pate et al., 1990; Knox and Clarke, 2005; Bowen and Pate, 2017). This strategy may require relatively greater investment in P-rich rRNA, and thus ribosomes, to support rapid protein synthesis and turnover, including replacement of damaged proteins (Raven, 2012). A high protein synthesis capacity may provide flexibility to acclimate to variable and changing environments (i.e. shallow sand over laterite or limestone, where water availability may fluctuate) and complete the life cycle quickly. Unlike B. sessilis, B. attenuata with larger seeds (Shi et al., 2020) and higher LMA has the ability to resprout from epicormic buds or lignotubers (Pate et al., 1991; Groom and Lamont, 2011), a strategy associated with a slower RGR (Pate et al., 1990; Knox and Clarke, 2005; Bowen and Pate, 2017). Thus, selection in B. attenuata was based on a lower investment in nucleic acid P, as well as the ability to allocate more biomass to deep roots compared with B. sessilis (Shi et al., 2020). Thus, it does not need to grow fast and complete its life cycle quickly (Pate et al., 1990; Knox and Clarke, 2005; Bowen and Pate, 2017).
The Pi concentration in slow-growing B. attenuata was higher than that in the faster growing B. sessilis when grown in sand and SLAT, slightly higher than when grown in SLIM. Cell vacuoles serve as a reservoir for excess Pi in most plants which can then be drawn upon as P availability decreases (Mimura, 1995). Changes in total foliar P concentration with Pi supply generally reflect the accumulation of Pi in vacuoles which is typically greater in slow-growing species than in fast-growing species (Güsewell, 2004). Thus, fast-growing species convert Pi into growth-sustaining organic P, rather than accumulating Pi, as in slow-growing species.
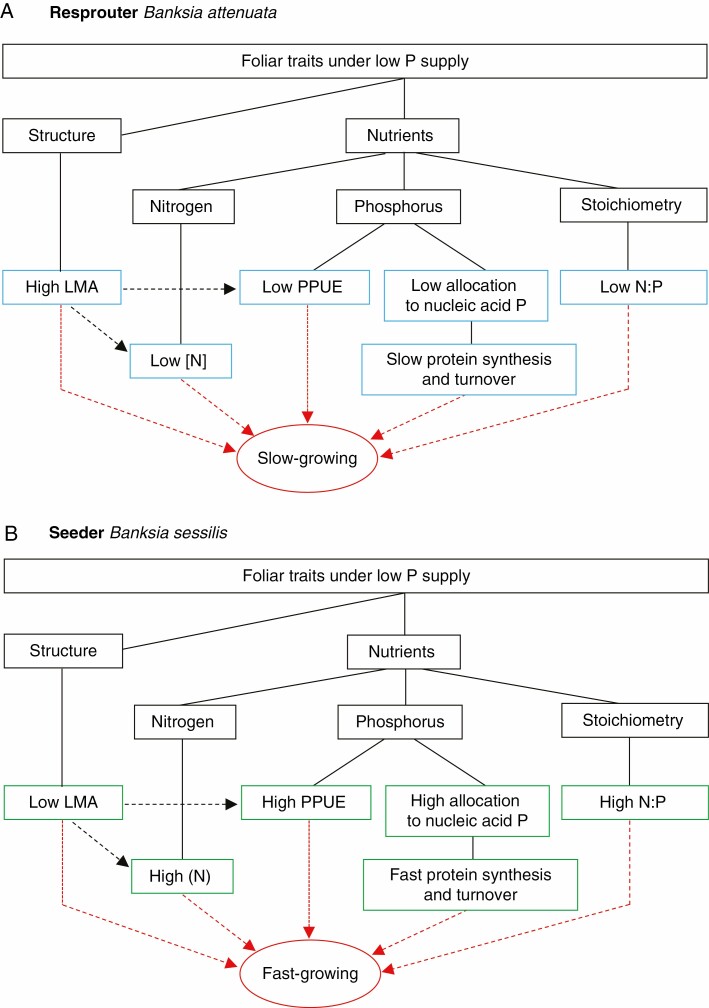
The metabolite P concentrations and the proportions of total P in metabolites for B. sessilis were significantly greater than those for B. attenuata for plants grown in SLIM. Moreover, the PPUE of B. sessilis was greater than that of B. attenuata grown on all substrates. Hidaka and Kitayama (2009) suggested that high PPUE is sustained by the allocation of a greater proportion of P to metabolic P (metabolite P + Pi) than to structural P, as we have shown here. In addition, B. sessilis had a lower LMA than B. attenuata on all substrates tested; however, B. attenuata had higher lipid P concentrations when grown in SLAT and SLIM in response to the lower P availability compared with sand alone. This finding was partially in line with a study that showed that the concentration of structural P is greater in slow-growing plants with high LMA than in fast-growing plants with low LMA (Villar et al., 2006). In other words, a greater proportion of nucleic acid P, a lower proportion of lipid P and a lower LMA in B. sessilis than in B. attenuata are all traits associated with a faster RGR and shorter leaf life span (Veneklaas et al., 2012). Overall, the unique foliar traits of the two species revealed different patterns of P allocation in response to soil P availability and associated with growth strategy that may define the ecological niches in which they are found (Fig. 6).
Fig. 6.
Diagrams summarizing foliar traits affecting relative growth rates for the resprouter Banksia attenuata (A) and the seeder Banksia sessilis (B). Solid lines indicate connections between factors; dashed arrows indicate factors that affect another factor; the red dashed arrow indicates that the factor affects the relative growth rate. P, phosphorus; LMA, leaf mass per area; PPUE, photosynthetic P use efficiency.
Conclusions
Both B. attenuata and B. sessilis exhibited a unique pattern of allocating P to different P fractions within the leaves under P limitation. Faster growth of B. sessilis was associated with greater allocation of P to nucleic acids than in the slower growing B. attenuata to support greater protein synthesis, which is likely to be needed for greater protein turnover associated with rapid growth rates. The observations that B. sessilis had a lower LMA and higher N concentration, PPUE, allocation of P to nucleic acids and N:P ratios than B. attenuata are possibly adaptive traits to growth in more severely P-impoverished soils, and may account for the different distributions of the two species. We surmise that P allocation patterns are probably the functional basis explaining why plants can reduce foliar P concentrations on P-impoverished soils. The foliar nutrient-allocation patterns and distinct foliar traits of the two Banksia species reveal different adaptive strategies in response to soil P availability and match their differences in growth strategies.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of Figure S1: the phosphorus concentration in soil solution extracted by citrate and iso-citrate for different soil substrates.
ACKNOWLEDGEMENTS
We thank Albina Ilyasova, Wenli Ding, Yunhe Wang, Asad Prodhan and Patrick E. Hayes for their assistance in aspects of this work, and Rob Creasy and Bill Piasini for help with maintaining the plants in the glasshouse.
FUNDING
This research was supported by an Australian Research Council Discovery Project grant (DP130100005) awarded to H.L. Z.H. was supported by China Scholarship Council Project grant (201508220115).
LITERATURE CITED
- Ames BN. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. vol. VIII. Complex carbohydrates. Methods in Enzymology 8: 115–118. [Google Scholar]
- Bowen BJ, Pate JS. 2017. Patterns of storage tissue and starch distribution in the young taproot of obligate seeders and resprouters of Australian Proteaceae (Juss.): possible evidence of homoplastic evolution. Austral Ecology 42: 617–629. [Google Scholar]
- Brooks A, Woo KC, Wong SC. 1988. Effects of phosphorus nutrition on the response of photosynthesis to CO2 and O2, activation of ribulose bisphosphate carboxylase and amounts of ribulose bisphosphate and 3-phosphoglycerate in spinach leaves. Photosynthesis Research 15: 133–141. [DOI] [PubMed] [Google Scholar]
- Chapin FS III, Kedrowski RA. 1983. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 64: 376. [Google Scholar]
- Clarke PJ, Lawes MJ, Midgley JJ, et al. . 2013. Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire. New Phytologist 197: 19–35. [DOI] [PubMed] [Google Scholar]
- Conroy JP, Milham PJ, Reed ML, Barlow EW. 1990. Increases in phosphorus requirements for CO2-enriched pine species. Plant Physiology 92: 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton MD, Veneklaas EJ, Freimoser FM, Lambers H. 2007. Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant, Cell & Environment 30: 1557–1565. [DOI] [PubMed] [Google Scholar]
- Elser JJ, Hamilton A. 2007. Stoichiometry and the new biology: the future is now. PLoS Biology 5: 1404–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH. 1996. Organism size, life history, and N:P stoichiometry: Toward a unified view of cellular and ecosystem processes. Bioscience 46: 674–684. [Google Scholar]
- Epstein E, Bloom AJ. 2005. Mineral nutrition of plants: principles and perspectives. Sunderland, MA: Sinauer. [Google Scholar]
- Fredeen AL, Raab TK, Rao IM, Terry N. 1990. Effects of phosphorus nutrition on photosynthesis in Glycine max (L.) Merr. Planta 181: 399–405. [DOI] [PubMed] [Google Scholar]
- Fujita K, Okada M, Lei K, et al. . 2003. Effect of P-deficiency on photoassimilate partitioning and rhythmic changes in fruit and stem diameter of tomato (Lycopersicon esculentum) during fruit growth. Journal of Experimental Botany 54: 2519–2528. [DOI] [PubMed] [Google Scholar]
- Geider R, La Roche J. 2002. Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. European Journal of Phycology 37: 1–17. [Google Scholar]
- Groom PK, Lamont BB. 2011. Regional and local effects on reproductive allocation in epicormic and lignotuberous populations of Banksia menziesii. Plant Ecology 212: 2003–2011. [Google Scholar]
- Güsewell S. 2004. N:P ratios in terrestrial plants: variation and functional significance. New Phytologist 164: 243–266. [DOI] [PubMed] [Google Scholar]
- Hayes PE, Turner BL, Lambers H, Laliberté E. 2014. Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. Journal of Ecology 102: 396–410. [Google Scholar]
- Hayes PE, Guilherme Pereira C, Clode PL, Lambers H. 2019. Calcium-enhanced phosphorus toxicity in calcifuge and soil-indifferent Proteaceae along the Jurien Bay chronosequence. New Phytologist 221: 764–777. [DOI] [PubMed] [Google Scholar]
- Herbert DA, Fownes JH. 1995. Phosphorus limitation of forest leaf area and net primary production on a highly weathered soil. Biogeochemistry 29: 223–235. [Google Scholar]
- Hidaka A, Kitayama K. 2009. Divergent patterns of photosynthetic phosphorus-use efficiency versus nitrogen-use efficiency of tree leaves along nutrient-availability gradients. Journal of Ecology 97: 984–991. [Google Scholar]
- Hidaka A, Kitayama K. 2011. Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on Mount Kinabalu, Borneo. Journal of Ecology 99: 849–857. [Google Scholar]
- Hidaka A, Kitayama K. 2013. Relationship between photosynthetic phosphorus-use efficiency and foliar phosphorus fractions in tropical tree species. Ecology and Evolution 3: 4872–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou E, Luo Y, Kuang Y, et al. . 2020. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems ecosystems. Nature Communications 11: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox KJE, Clarke PJ. 2005. Nutrient availability induces contrasting allocation and starch formation in resprouting and obligate seeding shrubs. Functional Ecology 19: 690–698. [Google Scholar]
- Kooyman RM, Laffan SW, Westoby M. 2017. The incidence of low phosphorus soils in Australia. Plant and Soil 412: 143–150. [Google Scholar]
- Kuppusamy T, Giavalisco P, Arvidsson S, et al. . 2014. Lipid biosynthesis and protein concentration respond uniquely to phosphate supply during leaf development in highly phosphorus-efficient Hakea prostrata. Plant Physiology 166: 1891–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Chapin FS III, Pons TL. 2006. Plant physiological ecology. New York: Springer. [Google Scholar]
- Lambers H, Brundrett MC, Raven JA, Hopper SD. 2010. Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant and Soil 334: 11–31. [Google Scholar]
- Lambers H, Cawthray GR, Giavalisco P, et al. . 2012. Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytologist 196: 1098–1108. [DOI] [PubMed] [Google Scholar]
- Lambers H, Ahmedi I, Berkowitz O, et al. . 2013. Phosphorus nutrition of phosphorus-sensitive Australian native plants: threats to plant communities in a global biodiversity hotspot. Conservation Physiology 1: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang G, Zhang R, Li L. 2016. A negative relationship between foliar carbon isotope composition and mass-based nitrogen concentration on the eastern slope of Mount Gongga, China. PLoS One 11: e0166958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusiewicz H, Golik B. 2004. Determination of major and trace elements in biological materials by microwave induced plasma optical emission spectrometry (MIP-OES) following tetramethylammonium hydroxide (TMAH) solubilization. Microchemical Journal 76: 23–29. [Google Scholar]
- Matzek V, Vitousek PM. 2009. N:P stoichiometry and protein:RNA ratios in vascular plants: an evaluation of the growth-rate hypothesis. Ecology Letters 12: 765–771. [DOI] [PubMed] [Google Scholar]
- McArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- Mimura T. 1995. Homeostasis and transport of inorganic phosphate in plants. Plant & Cell Physiology 36: 1–7. [Google Scholar]
- Parry GD. 1981. The meanings of r- and K-selection. Oecologia 48: 260–264. [DOI] [PubMed] [Google Scholar]
- Pate JS, Bell TL. 1999. Application of the ecosystem mimic concept to the species-rich Banksia woodlands of Western Australia. Agroforestry Systems 45: 303–341. [Google Scholar]
- Pate JS, Froend RH, Bowen BJ, Hansen A, Kuo J. 1990. Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of S.W. Australia. Annals of Botany 65: 585–601. [Google Scholar]
- Pate J, Meney K, Dixon K. 1991. Contrasting growth and morphological characteristics of fire-sensitive (obligate seeder) and fire-resistant (resprouter) species of Restionaceae (Shemisphere restiads) from south-western Western-Australia. Australian Journal of Botany 39: 505–525. [Google Scholar]
- Pate JS, Verboom WH, Galloway PD. 2001. Co-occurrence of Proteaceae, laterite and related oligotrophic soils: coincidental associations or causative inter-relationships? Australian Journal of Botany 49: 529–560. [Google Scholar]
- Prodhan MA, Jost R, Watanabe M, Hoefgen R, Lambers H, Finnegan PM. 2016. Tight control of nitrate acquisition in a plant species that evolved in an extremely phosphorus-impoverished environment. Plant, Cell & Environment 39: 2754–2761. [DOI] [PubMed] [Google Scholar]
- Rao IM, Fredeen AL, Terry N. 1989. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet: I. Changes in growth, gas exchange, and Calvin cycle enzymes. Plant Physiology 90: 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA. 2012. Protein turnover and plant RNA and phosphorus requirements in relation to nitrogen fixation. Plant Science 188–189: 25–35. [DOI] [PubMed] [Google Scholar]
- Reef R, Ball MC, Feller IC, Lovelock CE. 2010. Relationships among RNA:DNA ratio, growth and elemental stoichiometry in mangrove trees. Functional Ecology 24: 1064–1072. [Google Scholar]
- Reich PB, Uhl C, Walters MB, Ellsworth DS. 1991. Leaf lifespan as a determinant of leaf structure and function among 23 amazonian tree species. Oecologia 86: 16–24. [DOI] [PubMed] [Google Scholar]
- Seneweera SP, Conroy JP. 1997. Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorus nutrition. Japanese Society of Soil Science and Plant Nutrition 43: 1131–1136. [Google Scholar]
- Shane MW, Lambers H. 2005. Cluster roots: a curiosity in context. Plant and Soil 274: 101–125. [Google Scholar]
- Shi J, Strack D, Albornoz FE, Han Z, Lambers H. 2020. Differences in investment and functioning of cluster roots account for different distributions of Banksia attenuata and B. sessilis, with contrasting life history. Plant and Soil 447: 85–98. [Google Scholar]
- Sterner RW, Elser JJ. 2002. Ecological stoichiometry. Princeton, NJ: Princeton University Press. [Google Scholar]
- Sulpice R, Ishihara H, Schlereth A, et al. . 2014. Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species. Plant, Cell & Environment 37: 1276–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DS, Montagu KD, Conroy JP. 2006. Why does phosphorus limitation increase wood density in Eucalyptus grandis seedlings? Tree Physiology 26: 35–42. [DOI] [PubMed] [Google Scholar]
- Veneklaas EJ, Lambers H, Bragg J, et al. . 2012. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytologist 195: 306–320. [DOI] [PubMed] [Google Scholar]
- Villar R, Robleto JR, De Jong Y, Poorter H. 2006. Differences in construction costs and chemical composition between deciduous and evergreen woody species are small as compared to differences among families. Plant, Cell & Environment 29: 1629–1643. [DOI] [PubMed] [Google Scholar]
- Viscarra Rossel RA, Bui EN. 2016. A new detailed map of total phosphorus stocks in Australian soil. Science of the Total Environment 542: 1040–1049. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. . 2004. The world-wide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Yan L, Zhang X, Han Z, Pang J, Lambers H, Finnegan PM. 2019. Responses of foliar phosphorus fractions to soil age are diverse along a 2 Myr dune chronosequence. New Phytologist 223: 1621–1633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.