Figure 4.
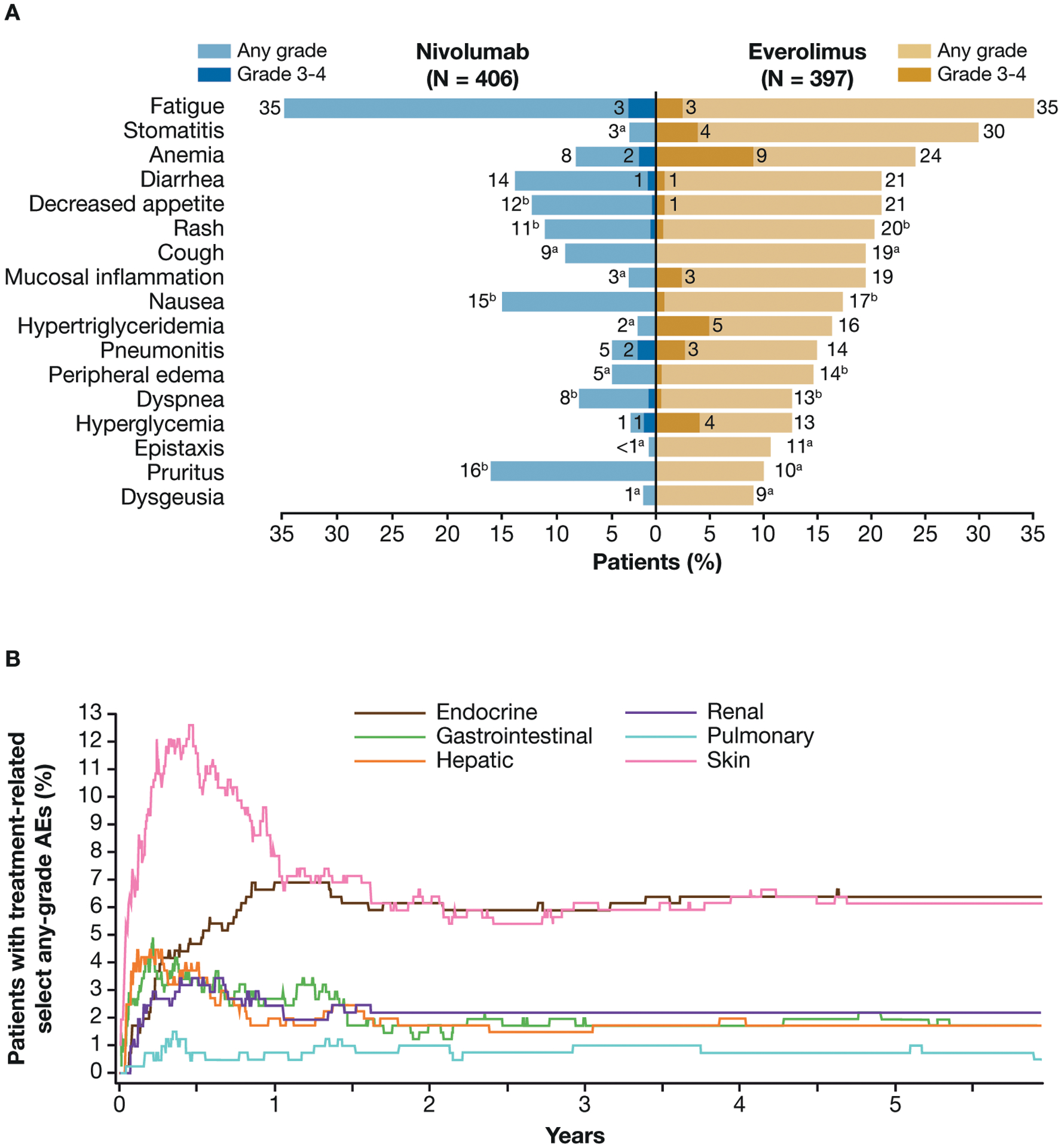
Treatment-related adverse events (AEs) reported in ≥10% of treated patients in either arm (A) and median time to onset and resolution of nivolumab-related select (immune-related) AEs of any grade (B).
aNo patient reported a grade 3–4 treatment-related AE.
b<1% of patients experienced a grade 3 or 4 treatment-related AE.
