Abstract
While plateau airway pressure alone is an unreliable estimate of lung overdistension inspiratory transpulmonary pressure (PL) is an important parameter to reflect it in patients with ARDS and there is no concensus about which computation method should be used to calculate it. Recent studies suggest that different formulas may lead to different tidal volume and PEEP settings. The aim of this study is to compare 3 different inspiratory PL measurement method; direct measurement (PLD), elastance derived (PLE) and release derived (PLR) methods in patients with multiple mechanical abnormalities. 34 patients were included in this prospective observational study. Measurements were obtained during volume controlled mechanical ventilation in sedated and paralyzed patients. During the study day airway and eosephageal pressures, flow, tidal volume were measured and elastance, inspiratory PLE, PLD and PLR were calculated. Mean age of the patients was 67 ± 15 years and APACHE II score was 27 ± 7. Most frequent diagnosis of the patients were pneumonia (71%), COPD exacerbation(56%), pleural effusion (55%) and heart failure(50%). Mean plateau pressure of the patients was 22 ± 5 cmH2O and mean respiratory system elastance was 36.7 ± 13 cmH2O/L. EL/ERS% was 0.75 ± 0.35%. Mean expiratory transpulmonary pressure was 0.54 ± 7.7 cmH2O (min: − 21, max: 12). Mean PLE (18 ± 9 H2O) was significantly higher than PLD (13 ± 9 cmH2O) and PLR methods (11 ± 9 cmH2O). There was a good aggreement and there was no bias between the measurements in Bland–Altman analysis. The estimated bias was similar between the PLD and PLE (− 3.12 ± 11 cmH2O) and PLE and PLR (3.9 ± 10.9 cmH2O) measurements. Our results suggest that standardization of calculation method of inspiratory PL is necessary before using it routinely to estimate alveolar overdistension.
Keywords: Transpulmonary pressure, Mechanical ventilation, Respiratory failure, Eosephageal pressure, ARDS, İntensive care
Introductıon
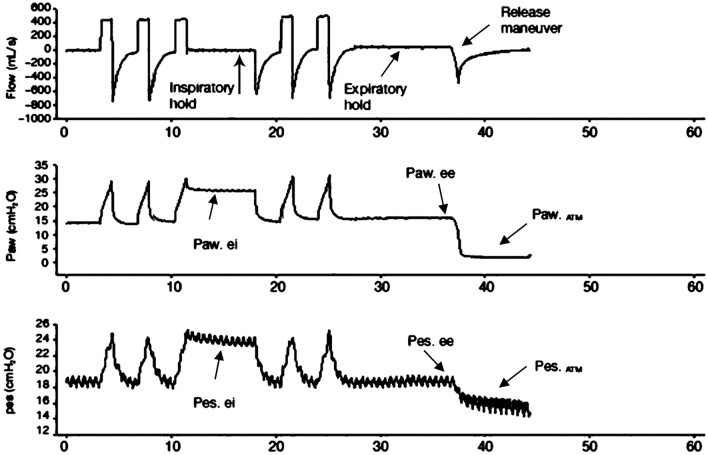
Mechanical ventilation (MV) is a life saving treatment modality in patients with respiratory failure. But unless perform appropriate settings according to patient requirements and thoraco-pulmonary mechanics it might cause ventilator induced lung injury (VILI) [1]. Current standard of care for ARDS includes limiting tidal volume (VT) and plateau pressures (Pplat). However, Pplat alone is an unreliable estimate of lung overdistension during MV especially in patients with increased chest wall elastance like chest wall edema, kyphoscoliosis, and intra-abdominal hypertension. In these situations pleural pressure increases and more force is necessary to distend the chest wall [2]. Because of this reason estimation of transpulmonary pressure (PL) is recommended [3]. Recent clinical studies suggest that adjusting ventilator settings based on PL measurements may improve oxygenation, and reduce VILI [3, 4]. The measurement of PL needs the estimation of pleural pressure(Ppl) from Pes [5–9]. PL is the real distending force of the lung parenchyma and in general it is calculated as the difference between the Pplat and the Ppl [5–11]. On the other hand there are different usage trends of PL in the treatment of ARDS patients. First, these measurements can be used to adjust PEEP. Increased chest wall elastance (ECW) elevates Ppl and may cause negative PL. As negative PL may lead to lung or airway collapse, adjusting PEEP to achieve positive end-expiratory PL prevents collapse and optimizes lung mechanics [3, 4, 11, 12]. In general for this aim expiratory PL is used. Second, PL measurements may be used monitor for lung overdistension and to account for the increased ECW during tidal breathing. For this aim measurement of inspiratory PL is recommended [3, 7]. On the other hand there are 3 different calculation method to find PL inspiratory and there is no consencus about which one should be used. Among the three approaches directly-measured method (PLD), is the most frequently used method in literature. With this method PL is simply calculated as the absolute difference between airway and Pes and inspiratory Pes is used for this aim [3]. Another frequently used method is the elastance-derived method (PLE) [2]. It estimates the end-inspiratory PL as the product of Pplat times the ratio between lung (EL) and respiratory system elastance (ERS). In this method delta Pes is used as Pes value. Third method is release-derived method (PLR) and is calculated as the difference between Paw and Pes from end-inspiration to atmospheric pressure and requires deconnection from the ventilator of the patient [13, 14 ](Fig. 1).
Fig. 1.
Representative tracings of airway and Pes used to calculate transpulmonary pressure with the different methods during controlled mechanical ventilation
Targeting an inaccurate PL could be potentially dangerous as it may lead to over- or underinflation of the lung and there is no adequate information about the yield of PL in general ICU patients with multiple mechanical abnormalities and which measurement method should be used [15]. In general studies related with transpulmonary pressure are performed in patients with ARDS or experimental ARDS models aiming to protect patients from VILI. On the other hand, ARDS patients consists of 7–10% of ICU patients and majority of medical ICU patients are patients with chronic cardiopulmonary diseases [16–18]. They may have atelectasis, edema, risk of overdistension also during mechanical ventilation. There are very few studies investigating other patients with respiratory failure and other etiologies than ARDS. The aim of this study is to compare 3 inspiratory PL measurement methods computed in patients with multiple mechanical abnormalities.
Materials and methods
Study population
The study was approved by the ethics committee of the Gazi University Faculty of Medicine (28.12.2015/166) and performed at ICU of Department of Pulmonary Critical Care Medicine of Gazi University School of Medicine. Before enrollement written informed consent was obtained by the patient or their family. The study population comprised 34 patients > 18 years old, with different pathologies. If the patient has a contraindication to esophageal catheterization (recent upper GI surgery, bleeding varices) then was excluded from the study.
Study design
All patients were intubated with an endotracheal tube no:8, sedated, paralyzed and kept in supine position. On the study day before the measurements propofol (10 mg/mL at a rate of 25–90 mg/h depending on need) was delivered intravenously and neuromuscular blocker was given till measurements were completed. The patients were ventilated with an AVEA ventilator (Viasys Health Care, Yorba Linda, CA). Baseline ventilator settings were; tidal volume 6 mL/kg, PEEP 5 cmH 2O, and respiratory rate 16 breaths/min. Respiratory rate was adjusted to maintain arterial carbon dioxide (PaCO2) between 35 and 45 cm H2O and FiO2 was set to maintain arterial oxygen saturation (%SO2) between 90 and 95%.
Eosephageal pressure (Pes) was measured with the AVEA ventilator and eosephageal balloon catheter (AVEA SmartCath Esophageal Pressure Monitoring Tube Set; 8 Fr adult. Viasys Health Care (Avea™; Carefusion)). First eosephageal ballon was positioned in the stomach to check the presence of positive deflection. Then, it was retracted until it reached the lower third of the esophagus(35–40 cm); in this position, an inspiratory occlusion was made to check for concordant changes in airway and esophageal pressure and inflated with 1.0–1.5 mL of air [8, 9, 19].
Measurement of respiratory mechanics
During an inspiratory and expiratory pauses, the airway and eosephageal pressures were measured. Following instrumentation, lung mechanics, peak (Ppeak), and end-inspiratory plateau (Pplatei) pressures; end-inspiratory esophageal pressure (Pesei) at Pplatei (PesPlat) at PEEP (PesPEEP) and at athmospheric pressure (PesZEEP) the difference between these two pressures (dPes); inspiratory PL pressures (cmH2O); arterial blood gas values (pH, PO2, PCO2) were recorded. Elastance of the respiratory system (ERS), lung (EL) and chest wall (ECW) during tidal inflation was calculated from the change in Pplat minus PEEP divided by the tidal volume ERS:
Definitions and calculations of the formulas
The following formulas were used for assessment of transpulmonary pressures and compared with each other. PLD is based on measured ‘absolute’ value of Pes. This method has an assumption that absolute Pes by itself can be used as a surrogate of Ppl and then PL is directly calculated as follows usng absolute values; Directly measured (PLD) = Pplat-Pes,ei where Pplat, Pes,ei are airway and esophageal pressure during an end-inspiratory occlusion [3, 20].
Elastance-derived method (PLE) does not require the measurement of Pes atmospheric pressure but simply the change in Pes: estimates the end-inspiratory PL as the product of plateau pressure times the ratio between lung (EL) and respiratory system elastance (ERS).
In release derived method (PLR) transpulmonary pressure is computed as the difference between Pplat and Pes from end-inspiratory to atmospheric pressure (Fig. 1).
Lastly we calculated accuracy values of PLE and PLR accepting PLD method as gold standard method. We accepted thereshold values for PLE as 25 cm H2O and for the others as 20 cmH2O for this analysis.
Statistical analysis
Data are reported as mean ± SD or as median(IQ), unless otherwise specified, as appropriate. Statistical significance was defined as p < 0.05. Baseline and physiologic variables were compared by Student’s t test for variables that were normally distributed and by Wilcoxon sign rank test for variables that were not normally distributed. To calculate correlations between the methods we used Pearson correlation coefficient. Two different formula were compared with Bland–Altman analysis(Linear regression and one sample t tests done for the Bland–Altman analyses). Accuracy of the different measurement methods were also calculated. SPSS version 20.0 (IBM, Armonk, NY, USA) was used for all statistical analyses and figures.
Results
Fourty-one patients were included in the study at the beginning but in 7 patients quality of measurements were not good and they were excluded from the study (In 2 patients we noticed later that eosephageal ballon was in the trachea. In 2 patients swinging of the eosephageal pressure tracing was not enough or there was no ossilation during the recordings and in 3 patients we noticed presence of spontaneous breathing during recordings). Demographics, diagnosis and comorbidities of the patients were given in Table 1. Admission blood gas values: pH: 7.30 ± 0.17, PaO2: 73 ± 28 mmHg:, PaCO2:52 ± 21 mmHg, HCO3:24 ± 6 mEq, Sat O2%: 87 ± 11%. According to statistical analysis results there were good correlation and agreement and there was no bias between the measurements. Elastance derived method measured PL significantly higher than other formulas(p < 0.05). Respiratory mechanics were presented in Table 2.
Table 1.
Baseline characteristics of the study population
| Age, years | 67 ± 15 |
| APACHE II | 27 ± 7 |
| Diagnosis of the patients | |
| Pneumonia | 24 (71%) |
| COPD exacerbation | 1 9(56%) |
| Heart failure | 17 (50%) |
| Acute renal failure | 13 (41%) |
| Acute respiratory distress sydrome | 10 (29%) |
| Pulmonary embolism | 6 (18%) |
| Pulmonary fibrosis | 9 (24%) |
| Atelectasis | 8 (24%) |
| Obesity hypoventilation sydrome | 8 (24%) |
| Pleural effusion | 18 (55%) |
| Chest Wall disease | 7 (23%) |
| Neurologic | 6 (18%) |
| Comorbidities | |
| COPD | 20 (59%) |
| Cardiac | 27 (79%) |
| Malignancy | 10 (29%) |
| Renal | 7 (21%) |
| Others | 10 (32%) |
Table 2.
Measurements of the airway and esophageal pressures and derived parameters
| Mean ± SD | Min Max | |
|---|---|---|
| Esephageal pressure, Pes end-insp plato, cmH2O | 10 ± 8 | − 4.29 |
| Esephageal pressure Pes end-exp, cmH2O | 7 ± 7 | − 6.23 |
| Esephageal pressure, Pespeak, cmH2O | 9 ± 9 | − 4.28 |
| Esephageal pressure, Pesplato, cmH2O | 10 ± 8 | − 1.5.29 |
| Esephageal pressure, Pesexp, cmH2O | 6 ± 7 | − 6.3 |
| Esephageal pressure, Pes ZEEP, cmH2O | 1.8 ± 4,3 | − 9.13 |
| Ppeak, cmH2O | 28 ± 6 | 17.38 |
| Plato pressure, Pplat, cmH2O | 22 ± 5 | 14.32 |
| Pexpiratory, cmH2O | 6 ± 3 | 0.5 16 |
| Elastance of respiratory system, ERS, cmH2O/L | 36.7 ± 13 | 20.79 |
| Elastance of lung, EL, cmH2O/L | 28.2 ± 17 | − 40.65 |
| Elastance of chest wall, ECW, cmH2O/L | 10.5 ± 16 | − 3.90 |
| EL/ERS,% | 0.75 ± 0.35 | − 0.80.1 |
| Inspiratory transpulmonary pressure, PLD direct method, cmH2O | 13 ± 9 | − 2.29 |
| Inspiratory transpulmonary pressure PLR released derived method, cmH2O | 11.4 ± 9,4 | − 4.1.28.7 |
| Inspiratory transpulmonary pressure, elastance derived PLE, cmH2O | 18 ± 9 | 2.45 |
| PLexpiratory, cmH2O, expiratory measurement | 0.54 ± 7.7 | − 21.12 |
| Mechanical ventilation parameters | ||
| PEEPi, cmH2O | 2.6 ± 3.1 | 0.5–13 |
| Tidal volume, VT, mL | 434 ± 38 | 354–520 |
| FiO2,% | 43 ± 15 | 40–100 |
| Respiratory rate, rate/min | 17 ± 4 | 12–31 |
| PEEP set, cmH2O | 5 | 5–5 |
Comparison of elastance derived method with directly measured method
There was a significant difference between the mean values of measurements according to Wilcoxon signed rank test results[13 ± 9 cmH2O for PLD and 18 ± 9 cmH2O for PLE, (p = 0.014)], and there was a good aggreement and there was no bias between the measurements Bias, (regression) in Bland–Altman analysis was: p: 0.833.
Agreement(one sample t test):
Mean difference: − 3.12 ± 11, p: 0.093, upper confidence limit: 18.44((11× 1.96) + (− 3.12)), lower confidence limit: − 18.44 (− (11 × 1.96)–(− 3.12)).
Correlation analysis showed good and significant correlation between the measurements (Spearmann correlation analysis: R: 0.619, p: 0.005).
Comparison of elastance derived method and relased derived method
Since release derived PL (PLR) calculated only in 26 patients all statistical analysis including Bland–Altman was performed in them.
There was a significant difference between the mean values of measurements (PLR 11 ± 9, PLE 15.2 ± 10, p: 0.005). In Bland–Altman analysis there was a good aggreement and there was no bias between the measurements (Bias (regression): p:0.776).
Agreement (One sample t test): Mean difference: 3.9 ± 10,9, p: 0.080. upper confidence limit: 25.26((10.9 × 1.96) + (3.9)) lower confidence limit: − 17.46) (− (10.9 × 1.96)- [3, 9]).
Correlation analysis showed good and significant correlation between the measurements (R:0.615, p: 0.002). Bland–Altman plots of the comparisons were given in Fig. 2.
Fig. 2.
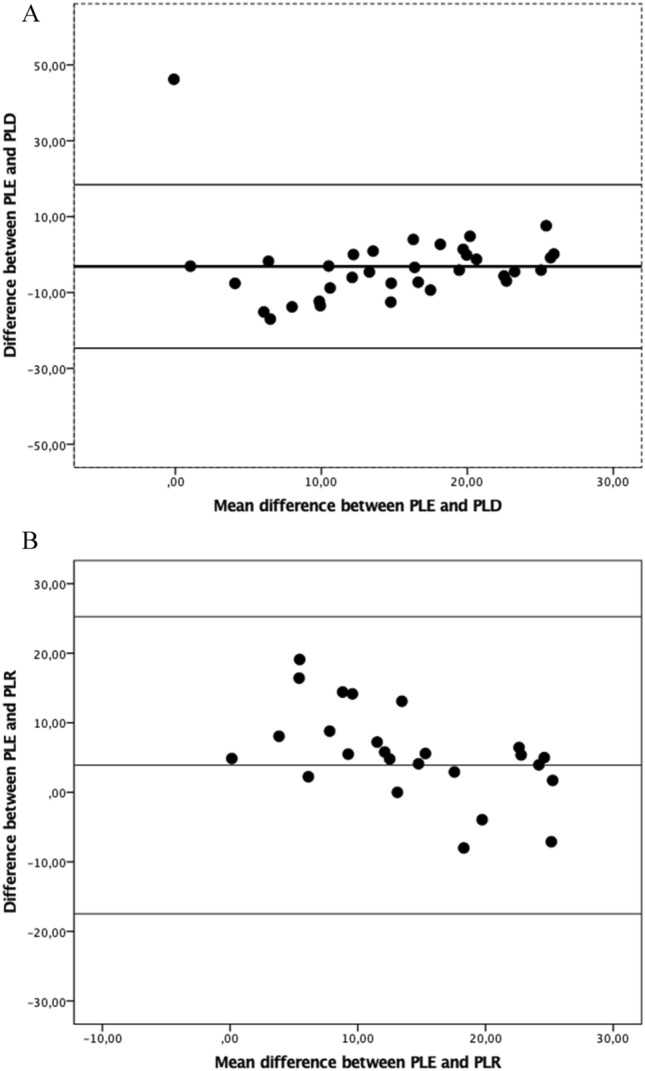
Comparison of different formulas with Bland–Altman graphic
Accuracy values of the PLE and PLR were 79% and 77% respectively when we consider PLD as gold standard method.
Discussion
Our study results showed that there was a good correlation and agreement between the measurements and there was no bias between them, but elastance derived method calculated PL significantly higher than the other two methods. These findings are in line with the results of other studies performed in patients with ARDS [21, 22]. Inspiratory PL is an important parameter to reflect lung overdistension and PLD is the most frequently used method fort his aim. But there is progressively increasing clinical data and debate about the differences between the PLD and PLE. Since there is no concensus about which computation method should be used to calculate PL recent literature have measured and presented the results of both measurement method in their studies. Nearly in all of them inspiratory PL calculated with PLE method is about 5 to 8 cmH2O higher than the PLD. In this clinical study we confirmed these results in our ICU patient population with mixed pulmonary mechanical problems. Computation method is important while using PL to set PEEP and tidal volume. Recent studies suggest that different formulas may lead different ventilator settings [23]. In a recent study comparing obese and nonobese COVID19 patients’ respiratory mechanics Mezidi et al. used both inspiratory PLD and PLE measurement methods. They found that PLE measured PL higher than PLD method in both obese and nonobese patients. At 16 cm H2O of PEEP, while 71% of non-obese and 0% of obese patients had PLD > 20 cm H2O, 86% of non-obese and 75% of obese patients had PLE > 20 cmH2O [21].
Berges’ et al. aimed to compare estimated alveolar distension with measurement of PLE, during individual PEEP titration using two different targets: Pplat 28–30 cmH2O or positive PLexpiratory 0–5 cmH2O [22]. They also monitored overdistension using PLD method. When they titrated PEEP levels according to EXPRESS protocol, PEEP was titrated on the basis of Pplat. Mean PLE was 20 cmH2O, and PLD was significantly lower than PLE with a mean difference of 8.5 cmH2O (p < 0.0001). With the PLexpi protocol, mean PLE was 24 cmH2O, and PLD was significantly lower than PLE with a mean of difference of 8.7 cmH2O (p < 0.0001).
One of the possible explanations of why these three different method give different estimates of PL is that they uses different Pes values to calculate PL. While absolute inspiratory Pes value is used to calculate PLD, delta Pes(difference Pes end-inspiratory and Pes end expiratory) is used to calculate PLE. On the other hand PLR uses Pes measured at PZEEP at atmospheric pressure during release maneuver. This method requires deconnection of patient from the ventilator [13, 14]. As previously shown by Pelosi et al. and recently confirmed by experimental and clinical studies there is not a single Ppl value among the thorax [24–28]. In these studies authors placed pleural catheters to the dependent and nondependent lung regions and compared Ppl with the Pes and showed that the static Ppl increases from nondependent to dependent regions in supine position, creating a vertical pressure gradient. In Tilmonts’ study PL values calculated using Pes were close to those obtained from the dependent pleural catheter but higher than those obtained from the nondependent pleural catheter both during expiration and inspiration in ventilated lung transplant recipients. During controlled ventilation, the absolute value of Pes was higher than the Ppl of nondependent lung regions and could therefore underestimate the highest level of lung stress in nondependent lung regions. In addition, the elastance-derived method seemed useful to prevent this pitfall. Results of these studies suggest that the direct approach gives a better estimation of expiratory and inspiratory PL of the dependent lung, therefore possibly useful to set PEEP, while the elastances ratio approach better estimates the inspiratory PL of the nondependent lung so that it should be used to set the appropriate tidal volume (or pressure support) and as a target to reduce VILI.
Therefore recent studies suggest that both approaches may give results for clinical application but with different meanings.
Talmor and colleagues, assuming that Pes equals the Ppl, proposed to set PEEP as to reach a positive PL at end expiration. The rationale of this approach is that being the Pes equal to Ppl, a negative PL corresponds to a collapsed lung. Setting PEEP values according to inspiratory PLD they avoided overdistention of lung. This approach was proposed and tested in humans and resulted in better compliance and oxygenation, but not in a decreased mortality when compared to the traditional FiO2/PEEP table approach [3]. A recent randomized controlled trial was unable to show any survival benefit using this approach compared to an empirical high PEEP-FiO2 titration table in patients with moderate to severe ARDS [20].
To eliminate the influence of the mediastinal weight and assuming that its influence is constant throughout the respiratory cycle, Gattinoni et al. proposed PLE method [2, 29]. This method assumes that the lung and respiratory system pressure–volume curves are linear in the range of PEEP and the tidal volume used in the clinical setting. The reason why this method does not use absolute Pes value to calculate PL is that while delta Ppl equals to delta Pes, absolute Ppl is not equal to absolute Pes in all lung regions. This method estimates the portion of Paw that is spent to inflate the lungs, and the portion that is required to move the chest wall, based on the relative contribution of EL and ECW to ERS. In general 30 cmH2O of Ppl in a general ARDS population is associated with a PL of approximately 21 cmH2O, being the average EL/ERS equal to 0.7. On the other hand, the ECW / ECW is not a fixed value, but may vary in this population from 0.2 to 0.8. For this reason, the resulting PL may range from approximately 10 to 28 cmH2O after applying 30 cmH2O to the whole respiratory system.
In another study Chiumello et al. compared 3 measurement method of PL and found PLE and PLR very similar in patients with ARDS. They showed that, there was a good correlation between the end-inspiratory PL calculated with the PLE and the release-derived methods. The mean PLE and PLR was 14.4 ± 3.7 and 14.4 ± 3.8 cm H2O at 5 cmH2O of PEEP and 21.8 ± 5.1 and 21.8 ± 4.9 cmH2O at 15 cmH2O of PEEP, respectively [13]. In contrast to this study we found PLE significantly higher than PLR in our study.
Approximately 30% of our study population had obesity and intraabdominal hypertension (IAH) in our study. Obesity increases baseline IAP of approximately 9–15 mmHg. Obese patients have reduced lung volume with increased atelectasis, lung and chest wall compliance. Based on previous studies’ data, Pelosi et al. found a relationship between IAP and chest wall elastance. They recommended that PLD measurement should be required if IAP is higher than 12 mmHg or if chest wall elastance is likely to be increased for other reasons [30, 31].
Assessment of PL seems important to understand the effect of different ventilator settings and to optimize interventions. Therefore before use it routinely for assessment of lung overdistension it is important to know how to measure it and which upper limit should be used needs to be determined and standardized. Despite all three methods reflect lung stress there are different reports giving acceptable upper limit of PL. In general limiting PLE lower than 25 cmH2O and PLD lower than 20 mmHg have been proposed in ARDS patientsy [5, 6].
Limitations of the study
Major limitation of our study is limited number of patients. Having more patients and to compare different calculation methods in patients with different pathologies such as obesity chest wall problems would have been more informative.
Conclusion
Since targeting an inaccurate PL could be potentially dangerous as it may lead to over- or underinflation of the lung and thus could cause VILI and every method for the estimation of PL is based on assumptions, the choice of which method and threshold value to use should be standardize. These issues need to be supported by further trials.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethics committee of the Gazi University Faculty of Medicine (28.12.2015/166).
Informed consent
Written informed consent was obtained from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 2.Gattinoni L, Chiumello D, Carlesso E, Valenza F. Bench-to-bedside review: chest wall elastance in acute lung injury/acute respiratory distress syndrome patients. Crit Care. 2004;8:350–355. doi: 10.1186/cc2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasso S, Terragni P, Birocco A, et al. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med. 2012;38:395–403. doi: 10.1007/s00134-012-2490-7. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida T, Grieco DL, Brochard L. Guiding ventilation with transpulmonary pressure. Insensive Care Med. 2019;45:535–538. doi: 10.1007/s00134-018-5483-3. [DOI] [PubMed] [Google Scholar]
- 6.Grieco DL, Chen L, Brochard L. Transpulmonary pressure: importance and limits. Ann Transl Med. 2017;5(14):285. doi: 10.21037/atm.2017.07.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talmor D, Sarge T, O'Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med. 2006;34:1389–1394. doi: 10.1097/01.CCM.0000215515.49001.A2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, Pelosi P, Talmor D. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Brochard L. Esophageal pressure monitoring: why, when and how? Curr Opin Crit Care. 2018;24:216–222. doi: 10.1097/MCC.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 10.Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178:346–355. doi: 10.1164/rccm.200710-1589OC. [DOI] [PubMed] [Google Scholar]
- 11.Marini JJ. Should we titrate positive end-expiratory pressure based on an end-expiratory transpulmonary pressure? Ann Transl Med. 2018;6(19):391. doi: 10.21037/atm.2018.08.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedler MO, Diktanaite D, Simeliunas E, Pilz M, Kalenka A. Prospective observational study to evaluate the effect of different levels of positive end-expiratory pressure on lung mechanics in patients with and without acute respiratory distress syndrome. J Clin Med. 2020;9:2446. doi: 10.3390/jcm9082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiumello D, Cressoni M, Colombo A, Babini G, Brioni M, Crimella F, Lundin S, Stenquist O, Gattinoni L. The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med. 2014;40:1670–1678. doi: 10.1007/s00134-014-3415-4. [DOI] [PubMed] [Google Scholar]
- 14.Umbrello M, Chiumello D. Interpretation of the transpulmonary pressure in the critically ill patient. Ann Transl Med. 2018;6(19):383. doi: 10.21037/atm.2018.05.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes-Puentes GA, Gard KE, Adams AB, Faltesek KA, Anderson CP, Dries DJ, Marini JJ. Value and limitations of transpulmonary pressure calculations during intra-abdominal hypertension. Crit Care Med. 2013;41:1870–1877. doi: 10.1097/CCM.0b013e31828a3bea. [DOI] [PubMed] [Google Scholar]
- 16.Bellani G, Laffey JG, Pham TI, Fan E, Brochard L, Esteban A, Gattinoni L, Haren FV, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, for the LUNG SAFE Investigators and the ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 17.Pham T, Rubenfeld GD. Fifty years of research in ARDS: the epidemiology of acute respiratory distress syndrome: a th birthday review. AJRCCM. 2017;195(7):860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 18.Pham T, Pesenti A, Bellani G, et al. Outcome of acute hypoxaemic respiratory failure: insights from the lung safe study. Eur Respir J. 2021;57(6):2003317. doi: 10.1183/13993003.03317-2020. [DOI] [PubMed] [Google Scholar]
- 19.Mietto C, Malbrain M, Chiumello D. Transpulmonary pressure monitoring during mechanical ventilation: a bench-to-bedside review. Anaesthesiol Intensive Ther. 2015;47:s27–s37. doi: 10.5603/AIT.a2015.0065. [DOI] [PubMed] [Google Scholar]
- 20.Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, Loring SH, Talmor D, for the EPVent-2 Study Group Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure–guided strategy vs an empirical high PEEP-FIO2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome a randomized clinical trial. JAMA. 2019;321(9):846–857. doi: 10.1001/jama.2019.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mezidi M, Daviet F, Chabert M, Hraiech S, Bitker L, Forel JM, Yonis H, Gragueb I, Dhelft F, Mapazian L, Richard JC, Guervilly C. Transpulmonary pressures in obese and nonobese COVID-19 ARDS Ann. Insensive Care. 2020;10:129. doi: 10.1186/s13613-020-00745-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergez M, Fritsch N, Tran-Van D, Saghi T, Bounkim T, Gentile A, Labadie P, Fontaine B, Ouattara A, Rozé H. PEEP titration in moderate to severe ARDS: plateau versus transpulmonary pressure. Ann Insensive Care. 2019;9:81. doi: 10.1186/s13613-019-0554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulati G, Novero A, Loring SH, et al. Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results. Crit Care Med. 2013;41:1951–1957. doi: 10.1097/CCM.0b013e31828a3de5. [DOI] [PubMed] [Google Scholar]
- 24.Pelosi P, Goldner M, McKibben A, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med. 2001;164(1):122–130. doi: 10.1164/ajrccm.164.1.2007010. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T, Amato MBP, Grieco DL, et al. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med. 2018;197:1018–1026. doi: 10.1164/rccm.201709-1806OC. [DOI] [PubMed] [Google Scholar]
- 26.Silva PL, Gama de Abre M. Regional distribution of transpulmonary pressure. Ann Transl Med. 2018;6(19):385. doi: 10.21037/atm.2018.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilmont A, Coiffard B, Yoshida T, et al. Oesophageal pressure as a surrogate of pleural pressure in mechanically ventilated patients. ERJ Open Res. 2021;7:00646–2020. doi: 10.1183/23120541.00646-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasticci I, Cadringher P, Giosa L, et al. Determinants of the esophageal–pleural pressure relationship in humans. J Appl Physiol. 2020;128:78–86. doi: 10.1152/japplphysiol.00587.2019. [DOI] [PubMed] [Google Scholar]
- 29.Gattinoni L, Giosa L, Bonifazi M, Pasticci I, Busana M, Macri M, Romitti F, Vassalli F, Quintel M. Targeting transpulmonary pressure to prevent ventilator-induced lung injury. Expert Rev Respir Med. 2019;13(8):737–746. doi: 10.1080/17476348.2019.1638767. [DOI] [PubMed] [Google Scholar]
- 30.Malbrain ML, Chiumello D, Pelosi P, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30(822–9):4. doi: 10.1007/s00134-004-2169-9. [DOI] [PubMed] [Google Scholar]
- 31.Pelosi P, Luecke T, Rocco PRM. Chest wall mechanics and abdominal pressure during general anaesthesia in normal and obese individuals and in acute lung injury. Curr Opin Crit Care. 2011;17:72–79. doi: 10.1097/MCC.0b013e3283427213. [DOI] [PubMed] [Google Scholar]