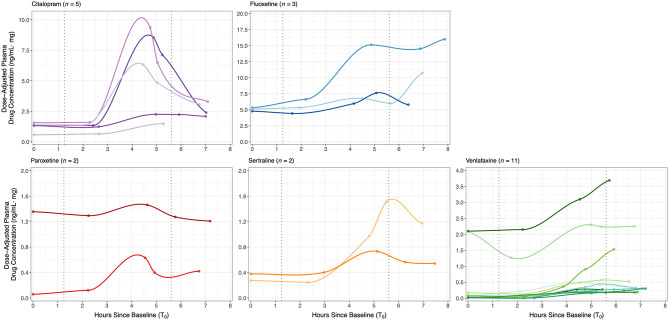
Figure 2.
Dose-adjusted plasma drug concentrations (ng/ml·mg) at baseline (T0) and four time-points post-dose across the study protocol for 23 SRI-treated mothers, grouped by antidepressant type. Concentration-time curves demonstrate inter-individual variability in SRI pharmacokinetics and maternal drug levels relative to the start of each fetal assessment; curves were fit to each individual's data with local polynomial regression. SRI oral dosing occurred a median of 1.83 h after T0. Median blood collection times were: baseline (T0) at 08h06, post-dose 1 (T1) at 10h21, post-dose 2 (T2) at 12h50, post-dose 3 (T3) at 13h38, and post-dose 4 (T4) at 14h57. Median start times of the baseline/pre-dose and post-dose fetal assessments were at a 1.3 and 5.6 h after T0, respectively (dotted vertical lines).