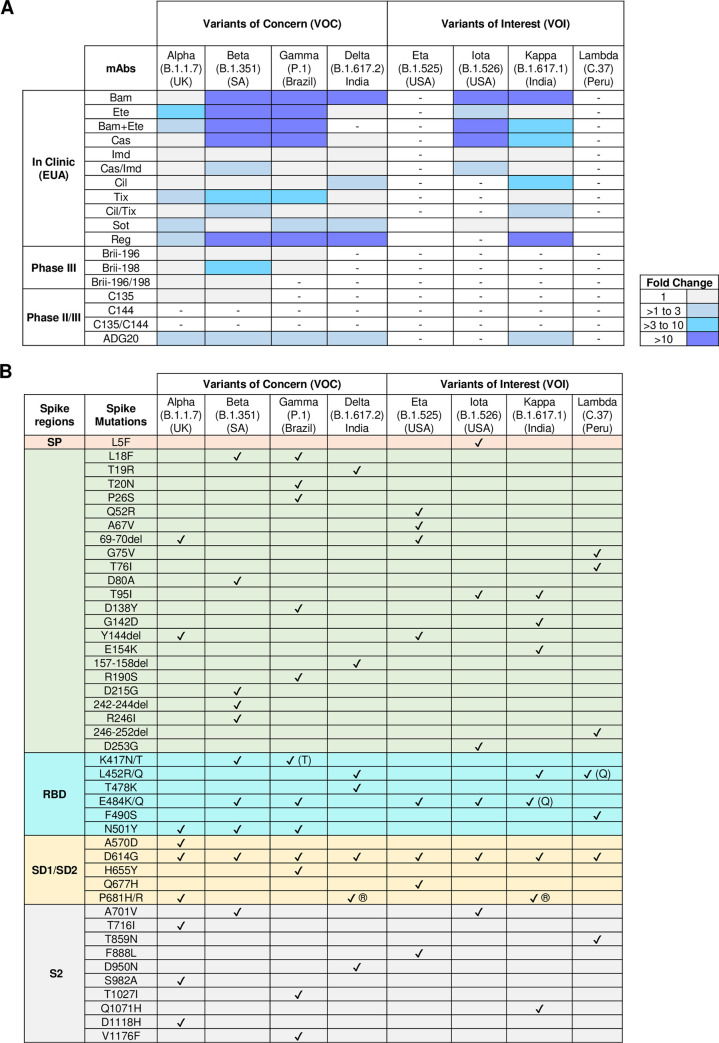
Fig 2. Neutralization potential of therapeutic mAbs against SARS-CoV-2 VOCs and VOIs.
(A) Neutralization potential of SARS-CoV-2 mAbs at various stages of development/clinic against VOCs and VOIs. Here, fold change represents the reduction in IC50 values of SARS-CoV-2 variant neutralization in comparison to wild-types virus. The abbreviations for mAbs in the clinic (EUA) are the following: Bam, Bamlanivimab (LY-CoV555); Ete, Etesevimab (LY-CoV016 or JS016 or CB6); Bam/Ete, Bamlanivimab+Etesevimab; Cas, Casirivimab (REGN10933); Imd, Imdevimab (REGN10987); Cas/Imd, Casirivimab+imdevimab (REGN-COV2); Cil, Cilgavimab (COV2-2130 or AZD1061); Tix, Tixagevimab (COV2-2196 or AZD8895); Tix/Cil, Tixagevimab+Cilgavimab; Sot, Sotrovimab (Vir-7831 or S309); Reg, Regdanvimab (CT-P59). (B) List of mutations present in the current SARS-CoV-2 VOCs and VOIs. EUA, emergency use authorization; mAb, monoclonal antibody; RBD, receptor-binding domain; S2, S2 subunit; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SD1, subdomain 1; SD2, subdomain 2; SP, signal peptide; VOC, variant of concern; VOI, variant of interest.