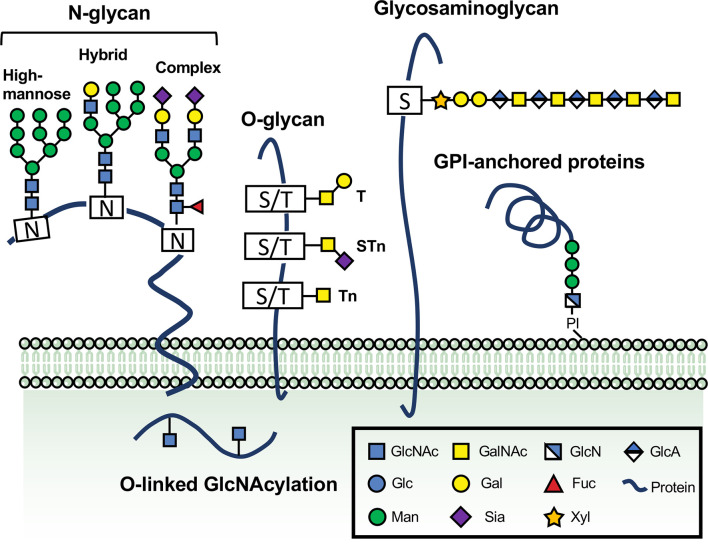
Figure 1.
The vital roles of glycosylation in physiological and pathological processes. Glycans are formed by the attachment of saccharides or sugar chains to proteins and lipids. A great number of naturally occurring glycans are abundant on the surface and cytoplasm of the cells. The main classes of glycoproteins consist of at least five major types, N-glycans, O-glycans, glycosaminoglycan, GPI-anchored proteins, and O-linked GlcNAcylation. N-glycans are generally considered the critical type of the cell surface glycoproteins because of their direct implications in cellular recognition and signaling transduction. They are covalently linked to asparagine (Asn) residues of polypeptide backbone via nitrogen linkages. N-glycans are divided into high-mannose, hybrid and complex types. Another common type of glycans involved in regulating immune responses and controlling cell metabolism is O-glycan, which can be further extended into long chains. A single O-GlcNAc addition to Ser/Thr residues catalyzed through OGT or EGF domain-specific O-linked GlcNAc transferase (EOGT) is mostly found on extracellular or intracellular compartments. Glycosylphosphatidylinositol (GPI) anchor is a molecule composed of a glycan linked to a polypeptide chain. Glycosaminoglycans (GAGs) represent a significant type of glycoproteins that are defined by the presence of long disaccharide repeats and further classified according to the composition of their repeating units. The glycosaminoglycans and GPI anchor are also depicted.