Abstract
Physical exercise leads to structural changes in the brain. However, it is unclear whether the initiation or continuous practice of physical exercise causes this effect and whether brain connectivity benefits from exercise. We examined the effect of 6 months of exercise on the brain in participants who exercise regularly (n = 25) and in matched healthy controls (n = 20). Diffusion tensor imaging brain scans were obtained from both groups. Our findings demonstrate that regular physical exercise significantly increases the integrity of white matter fiber tracts, especially those related to frontal function. This implies that exercise improves brain connectivity in healthy individuals, which has important implications for understanding the effect of fitness programs on the brains of healthy subjects.
Keywords: connectivity, diffusion tensor imaging, fractional anisotropy, longitudinal, physical exercise
1. Introduction
Aging is associated with changes in grey matter (GM) and white matter (WM) microstructures, resulting in cognitive decline and an increased risk of neurodegenerative brain conditions.[1] As the average age of adult populations is increasing throughout the world, more attention is being paid to lifestyle factors that preserve the cognitive vitality of older. One of these lifestyle factors is physical activity (PE).[2–4] PE may help maintain, or even enhance, cognitive and brain function across the lifespan. Although the general physiology of PE has been an active area of research for upwards of 4 decades, until recently, few studies have examined its neurocognitive effects.[5–7]
Considerable evidence shows that PE may enhance structural and functional modifications in the brain, influence cognitive functions, and provide psychological and biological benefits.[7–9] Cumulative research from animal studies has demonstrated that, at the cellular level, intensive motor training increases neurogenesis, synaptogenesis, gliogenesis, and angiogenesis in the hippocampus, neocortex, and cerebellum.[10–15] At the molecular level, PE can modulate neurotransmission systems such as serotonin, noradrenalin, and acetylcholine,[16,17] induce the release of the brain-derived neurotrophic factor brain-derived neurotrophic factor[18,19]; and the insulin-like growth factor-1,[20] and improve spatial memory performance.[21,22]
In humans, a growing body of evidence suggests that PE can positively influence brain plasticity.[5,22,23] PE has been reported to influence GM structure.[16,17] Increased volume of the cortex,[24–26] and hippocampus,[27,28] has been observed directly after PE in healthy individuals. However, other studies have failed to show that PE has a significant effect on, for example, cortical architecture.[19,20] PE facilitates the release of peripheral brain-derived neurotrophic factor,[29] which is essential to neural plasticity. PE enhances executive function and prevents age-related cognitive decline.[30,31] A recent systematic review of neuroimaging studies investigating the impact of PE on young people's brain structure and cognitive function[32] found that PE might induce changes in WM integrity and activate brain regions that are critical to cognitive processes.
However, the degree to which PE can modulate or enhance WM connectivity in healthy subjects who exercise regularly is not yet completely clear.
Therefore, the purpose of this study was to investigate the effect of PE on brain connectivity in healthy individuals. We aimed to assess brain changes in 2 groups. One group engaged in daily aerobic and anaerobic exercise for 6 months; the second group did not do any PE. We measured brain connectivity using diffusion tensor imaging (DTI) at 3 Tesla at baseline (time point 1) and then after the 6-month intervention (time point 2).
2. Materials and methods
A total of 45 subjects, divided into the exercise group (n = 25) and the non-exercise group (n = 20), were included in the study. Participants were between 19 and 27 years of age (Table 1); the groups were matched for age. All participants were males. All subjects provided written informed consent prior to participation in the study. All included individuals were physically healthy, meaning that they showed no evidence of significant cardiovascular, neuromuscular, endocrine, or another somatic disorders. None of the participants had a primary diagnosis of alcohol or substance abuse or dependence. All participants in the control group were physically inactive, defined as engaging in less than 1 hour of moderate PE weekly.
Table 1.
Subject characteristics.
| Age (years) | 23.3 ± 4.22 |
| Gender | 45M |
| Mini-Mental State Examination | 30 |
M = male.
2.1. Exercise group
Aerobic exercise was performed using an upright bicycle ergometer (starting with warm-up for 2 minutes, followed by cycling with tolerable workload for 4 minutes, then 2 minutes for cooling down), a recumbent bicycle ergometer (2 minutes of cycling for warming up at a comfortable pace with low resistance, followed by 4 minutes of cycling at high tolerable resistance, and finally, 2 minutes pace back to a comfortable level at low resistance for cooling down), a rowing machine (2 minutes warm up at a low intensity with the aim for 15–20 strokes per minute, followed by 4 minutes of rowing at high intensity with the aim for reaching 15–20 strokes per minute, then 2 minutes cool down at a low intensity), a cross trainer (2 min warm up at a low intensity with the aim for 40–50 stride per minute, followed by 4 minutes at high to reach 60–80 stride per minute, then cool down at a low resistance for 2 minutes) and a treadmill (2 minutes warm up by walking, then 4 minutes easy run at tolerable speed and finally cool down by regular walking for 2 minutes). Anaerobic exercise consisted of weight training (6 exercises per week, 3 sets, 10–15 repetitions; exercises engaged the biceps, triceps, abdominal muscles, quadriceps, pectoral muscles, and deltoid muscles).
2.2. Non-exercise group
All participants in the control group were physically inactive, defined as participating in less than 1 hour of moderate-intensity or vigorous-intensity aerobic physical exercise or an equivalent combination of both types throughout the week.[33]
2.3. Brain imaging
Brain scans were obtained using a 3 Tesla Siemens Medical Scanner at the KSU Medical Center, Riyadh, Saudi Arabia. High-resolution T1-weighted imaging was acquired using a multishot turbo field echo pulse sequence.
2.4. Postprocessing
2.4.1. Diffusion tensor image analysis
We performed voxel-wise analyses of fractional anisotropy (FA) maps across subjects using tract-based spatial statistics for the data from participants in the exercise group and the control group. First, the data were pre-processed for motion artifacts and distortions due to eddy currents. Non-brain tissue was removed using the brain extraction tool in the FMRIB Software Library (FSL) package. DTI data were calculated for each voxel after the diffusion tensor model was fitted to each voxel using FMRIB's diffusion toolbox from the FSL package. Next, we generated a subject-wise mid-space template, which was aligned to the FSL standard FA template non-linearly and then averaged to generate a study-specific mean FA map. This mean image was thresholded to an FA value of 0.2 and skeletonized to generate a WM tract skeleton representing the center of the tracts common to all subjects. Next, the values of axial diffusivity (AD) and radial diffusivity (RD) were mapped onto the skeleton using the projection vectors from each individual's FA-to-skeleton transformation.[33]
2.4.2. Statistical analyses
The 2 groups (exercise vs control) were compared using 2 sample t test in each voxel with threshold-free cluster enhancement, which is a statistical method for finding clusters without having to define clusters in a binary way, thereby cluster-like structures are enhanced but the image remains fundamentally voxelwise. By controlling the family-wise error rate, P values less than .05 were accepted to be significant, which means it has 95% confidence of no false positives in case of FA images, while P values less than .01 were accepted to be significant in case of AD and RD image (see FSL homepage; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise/UserGuide).
3. Results
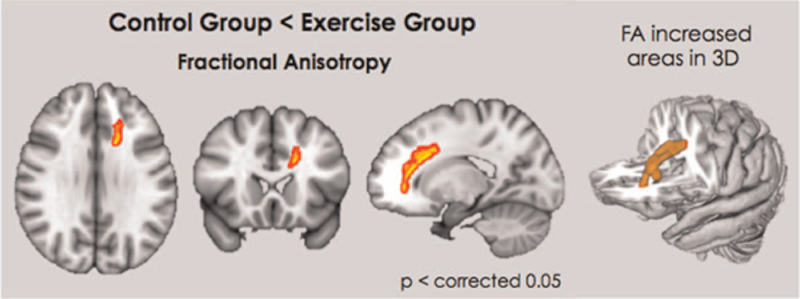
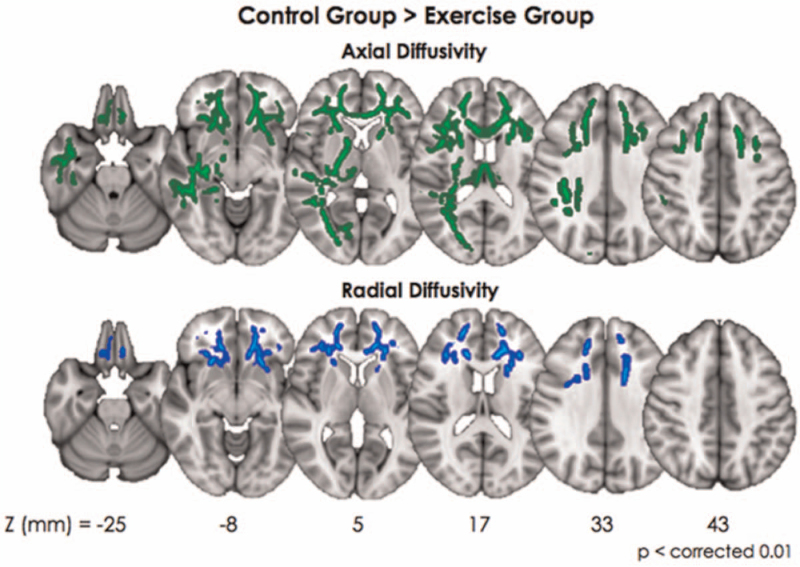
The exercise group showed significantly increased FA in the left frontal WM, including the genu portion of the corpus callosum (CC) and the anterior cingulum area (Fig. 1). The exercise group showed significantly less AD in most of the bilateral frontotemporal WM with the fornix, medial thalamus, and sensorimotor fibers (Fig. 2a, green). RD was decreased only in the frontal WM in the exercise group (Fig. 2b, blue).
Figure 1.
Exercise group showed significantly increased FA in left frontal white matter including genu portion of corpus callosum, anterior cingulum area. FA = fractional anisotropy.
Figure 2.
In case of axial diffusivity, exercise group showed significantly smaller value in most of the bilateral frontotemporal white matter with fornix and medial thalamus and sensorimotor fibers (Fig. 2a, green color). Contrast to axial diffusivity, the radial diffusivity was decreased only in frontal white matter (Fig. 2b, blue color).
4. Discussion
The findings of this study suggest that regular PE affects brain connectivity in healthy individuals. Longitudinal tract-based spatial statistics analyses revealed increased FA in the left frontal WM, including the genu portion of the CC and the anterior cingulum area. AD decreased in most of the bilateral frontotemporal WM with the fornix, medial thalamus, and sensorimotor fibers. RD also decreased in the frontal WM. These findings imply that an hour of daily exercise over a period of 6 months can enhance WM integrity, particularly in the fiber tracts.
Higher FA in the CC and bilateral frontotemporal WM indicates more efficient sensorimotor and cognitive communication between the 2 cerebral hemispheres.[34,35] Higher FA has also been shown to correlate with better functional connectivity between distant brain GM regions using resting-state functional magnetic response imaging,[36,37] and is further associated with improved cognitive function.[38,39]
Significant positive impacts on both the CC and frontotemporal fasciculi are consistent with previous studies that showed PE-induced increases in FA values in the corticospinal tract, superior longitudinal fascicle, inferior longitudinal fascicle, inferior fronto-occipital fascicle, and anterior thalamic radiation and in the body and splenium of the CC,[40] along with decreased RD.[31] Similarly, in another study, an 8-month PE intervention increased FA in the bilateral uncinated fasciculus and decreased RD in the left uncinated fasciculus compared to the control group.[8] A recent study by Rodriguez-Ayllon et al[24] showed a similar pattern of association; PE correlated positively with global FA and negatively with global mean diffusivity.[32] However, contrary to our results, another recent study found that aerobic exercise had no effect on WM microstructure in the aging brain and was not associated with DTI measures of either fraction anisotropy or mean diffusivity.[33] However, this might be attributed to the small sample size and large age range (from 57–86 years) and less robust DTI scanning protocol in that study.
A possible explanation for the PE-induced WM microstructural alterations found in our study might be that PE increases the flow of oxygen-rich blood in the neural circuits of the brain, thereby enhancing multiple exercise-mediated physiological mechanisms underpinning neuroprotective and neuroplastic processes in brain structures.[34–36]
Our results indicate that a 6-month intervention of combined aerobic and anaerobic exercise in healthy adults was associated with improvements in FA and diffusivity measures of WM microstructure. These findings suggest that physical training of an overlearned skill can continue to improve the structural connectivity of the brain in healthy individuals. Our findings also contribute to the growing literature on the benefits of PE for brain health[11,39] and for the prevention of diseases such as schizophrenia[8] and early-onset Alzheimer disease.[3]
One major strength of this study is that it provides evidence that PE modulates structural brain connectivity in healthy adults. It builds on previous studies by employing a combination of exercise interventions that includes aerobic and anaerobic exercise; this is useful as each type of exercise has different physiological mechanisms that contribute to neural plasticity.
However, the present study has several limitations that confine the generalizability of the current results. First, a convenience sampling was employed in this study instead of random sampling. Second, the study did not include a control for different types of PE. Finally, different training durations were not compared. Future research should include longitudinal studies with large sample sizes that investigate PE-induced changes in brain structures using multiple neuroimaging measures. In conclusion, continuous, regular exercise improves WM connectivity in the brain. The current findings have important implications for understanding the effect of fitness programs on the brains of healthy individuals and of patients with a range of conditions.
Acknowledgment
The authors extend their appreciation to participant who took part in the study.
Author contributions
Shahid Bashir, Fahad Al-Sultan and Abdulah Abu Jamea contributed to the conception and design of the research. Fahad Al-Sultan, Abdulah Abu Jamea, Abdullah Almousa, Mohammed Alnafisah, Maha Alzahrani acquired the data. Turki Abualait, Woo-Kyoung Yoo performed statistical analyses. Shahid Bashir drafted the manuscript. All authors critically revised the article and approved the final version of the manuscript.
Conceptualization: Shahid Bashir, Fahad Al-Sultan, Woo-Kyoung Yoo.
Data curation: Shahid Bashir, Fahad Al-Sultan, Abdulah Abu Jamea, Abdullah Almousa, Mohammed Alnafisah.
Formal analysis: Fahad Al-Sultan.
Investigation: Shahid Bashir, Fahad Al-Sultan, Abdulah Abu Jamea, Woo-Kyoung Yoo.
Methodology: Shahid Bashir, Fahad Al-Sultan, Abdullah Almousa, Maha Alzahrani, Turki Abualait.
Supervision: Shahid Bashir.
Visualization: Shahid Bashir, Abdulah Abu Jamea, Woo-Kyoung Yoo.
Writing – original draft: Shahid Bashir, Fahad Al-Sultan, Abdulah Abu Jamea, Abdullah Almousa, Mohammed Alnafisah, Maha Alzahrani, Turki Abualait, Woo Kyoung Yoo.
Writing – review & editing: Shahid Bashir, Fahad Al-Sultan, Abdulah Abu Jamea, Abdullah Almousa, Mohammed Alnafisah, Maha Alzahrani, Turki Abualait, Woo-Kyoung Yoo.
Footnotes
Abbreviations: AD = axial diffusivity, CC = corpus callosum, DTI = diffusion tensor imaging, FA = fractional anisotropy, FSL = FMRIB Software Library, GM = grey matter, PE = physical activity, RD = radial diffusivity, WM = white matter.
How to cite this article: Bashir S, Al-Sultan F, Jamea AA, Almousa A, Alnafisah M, Alzahrani M, Abualait T, Yoo WK. Physical exercise keeps the brain connected by increasing white matter integrity in healthy controls. Medicine. 2021;100:35(e27015).
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding this work through the research group no. (RGP- 1438-008).
The study was approved by local ethic committee of King Saud University, Saudi Arabia.
The consent was obtained before study. Written informed consent was obtained from the patient for publication of this paper and accompanying images.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 2003;14:125–30. [DOI] [PubMed] [Google Scholar]
- [2].Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008;300:1027–37. Erratum in: JAMA. 2009;301(3):276. [DOI] [PubMed] [Google Scholar]
- [3].Barber SE, Clegg AP, Young JB. Is there a role for physical activity in preventing cognitive decline in people with mild cognitive impairment? Age Ageing 2012;41:05–8. [DOI] [PubMed] [Google Scholar]
- [4].Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 2006;61:1166–70. [DOI] [PubMed] [Google Scholar]
- [5].Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci 2008;28:7031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lustig C, Shah P, Seidler R, Reuter-Lorenz PA. Aging, training, and the brain: a review and future directions. Neuropsychol Rev 2009;19:504–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burdette JH, Laurienti PJ, Espeland MA, et al. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci 2010;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Holzschneider K, Wolbers T, Röder B, Hötting K. Cardiovascular fitness modulates brain activation associated with spatial learning. Neuroimage 2012;59:3003–14. [DOI] [PubMed] [Google Scholar]
- [9].Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature 2004;427:311–2. [DOI] [PubMed] [Google Scholar]
- [10].Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 2011;108:3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Falkai P, Malchow B, Wobrock T, et al. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: a randomized controlled MRI study. Eur Arch Psychiatry Clin Neurosci 2013;263:469–73. [DOI] [PubMed] [Google Scholar]
- [12].Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry 2010;67:133–43. [DOI] [PubMed] [Google Scholar]
- [13].Scheewe TW, van Haren NE, Sarkisyan G, et al. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol 2013;23:675–85. [DOI] [PubMed] [Google Scholar]
- [14].Taubert M, Draganski B, Anwander A, et al. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 2010;30:11670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gondoh Y, Sensui H, Kinomura S, et al. Effects of aerobic exercise training on brain structure and psychological well-being in young adults. J Sports Med Phys Fitness 2009;49:129–35. [PubMed] [Google Scholar]
- [16].Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci 2009;12:1370–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Steele CJ, Bailey JA, Zatorre RJ, Penhune VB. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J Neurosci 2013;33:1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gärtner H, Minnerop M, Pieperhoff P, et al. Brain morphometry shows effects of long-term musical practice in middle-aged keyboard players. Front Psychol 2013;4:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Filley CM, Fields RD. White matter and cognition: making the connection. J Neurophysiol 2016;116:2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weinberg RS, Gould D. Foundations of Sport and Exercise Psychology. 6th ednChampaign, IL: Human Kinetics; 2015. [Google Scholar]
- [21].Fernandes J, Arida RM, Gomez-Pinilla F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci Biobehav Rev 2017;80:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mandolesi L, Polverino A, Montuori S, et al. Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front Psychol 2018;9:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mandolesi L, Gelfo F, Serra L, et al. Environmental factors promoting neural plasticity: insights from animal and human studies. Neural Plast 2017;2017:7219461.doi: 10.1155/2017/7219461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rodriguez-Ayllon M, Derks IP, van den Dries MA, et al. Associations of physical activity and screen time with white matter microstructure in children from the general population. NeuroImage 2020;205:116258. [DOI] [PubMed] [Google Scholar]
- [25].Clark CM, Guadagni V, Mazerolle EL, et al. Effect of aerobic exercise on white matter microstructure in the aging brain. Behav Brain Res 2019;373:112042. [DOI] [PubMed] [Google Scholar]
- [26].Valkenborghs SR, Noetel M, Hillman CH, et al. The impact of physical activity on brain structure and function in youth: a systematic review. Pediatrics 2019;144:e20184032. [DOI] [PubMed] [Google Scholar]
- [27].Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med 2018;52:154–60. [DOI] [PubMed] [Google Scholar]
- [28].Gazzaniga MS. Principles of human brain organization derived from split-brain studies. Neuron 1995;14:217–28. [DOI] [PubMed] [Google Scholar]
- [29].Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M. Callosal contributions to simultaneous bimanual finger movements. J Neurosci 2008;28:3227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].van den Heuvel M, Mandl R, Kahn RS, Pol H. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 2009;30:3127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lowe MJ, Beall EB, Sakaie KE, et al. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum Brain Mapp 2008;29:818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Madden DJ, Spaniol J, Costello MC, et al. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci 2009;21:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bull Fiona C, Al-Ansari Salih S, Biddle Stuart, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. British J Sports Med 2020;54:1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Svatkova A, Mandl RC, Scheewe TW, Cahn W, Kahn RS, Hulshoff Pol HE. Physical exercise keeps the brain connected: biking increases white matter integrity in patients with schizophrenia and healthy controls. Schizophr Bull 2015;41:869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chaddock-Heyman L, Erickson KI, Kienzler C, et al. Physical activity increases white matter microstructure in children. Front Neurosci 2018;12:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schaeffer DJ, Krafft CE, Schwarz NF, et al. An 8-month exercise intervention alters frontotemporal white matter integrity in overweight children. Psychophysiology 2014;51:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Scholey AB, Moss MC, Neave N, Wesnes K. Cognitive performance, hyperoxia, and heart rate following oxygen administration in healthy young adults. Physiol Behav 1999;67:783–9. [DOI] [PubMed] [Google Scholar]
- [38].Dietrich A, Sparling PB. Endurance exercise selectively impairs prefrontal-dependent cognition. Brain Cogn 2004;55:516–24. [DOI] [PubMed] [Google Scholar]
- [39].Radak Z, Hart N, Sarga L, et al. Exercise plays a preventive role against Alzheimer's disease. J Alzheimers Dis 2010;20:777–83. [DOI] [PubMed] [Google Scholar]
- [40].Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis 2013;57:47–55. [DOI] [PubMed] [Google Scholar]