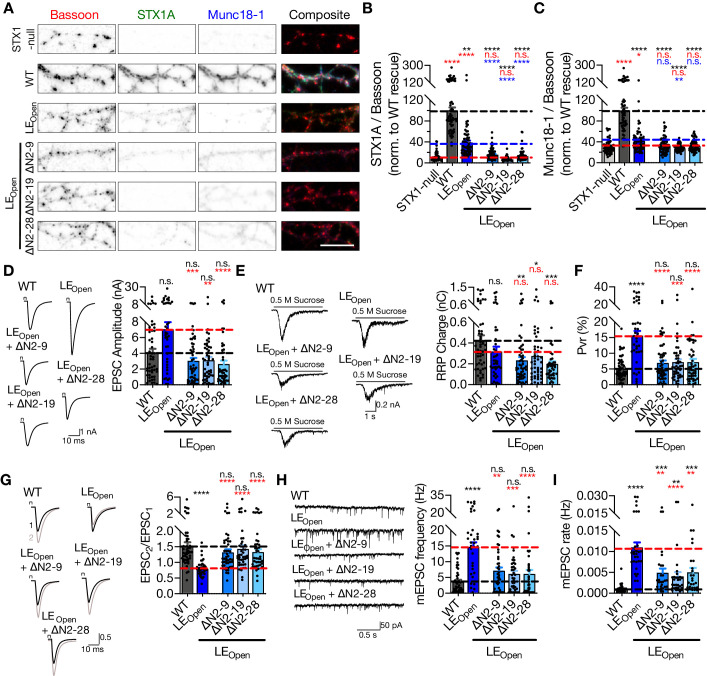
Figure 4. ‘Opening’ of STX1A in combination with the deletion of its entire N-terminal stretch does not impair neurotransmitter release.
(A) Example images of immunofluorescence labeling for Bassoon, STX1A, and Munc18-1 shown as red, green, and blue, respectively, in the corresponding composite pseudocolored images obtained from high-density cultures of STX1-null hippocampal neurons either not rescued or rescued with STX1AWT, STX1ALEOpen, STX1ALEOpen + ∆N2-9, STX1ALEOpen + ∆N2-19, or STX1ALEOpen + ∆N2-28. Scale bar: 10 µm (B, C) Quantification of the immunofluorescence intensity of STX1A and Munc18-1 as normalized to the immunofluorescence intensity of Bassoon in the same ROIs as shown in (A). The values were then normalized to the values obtained from STX1AWT neurons. (D) Example traces (left) and quantification of the amplitude (right) of EPSCs obtained from hippocampal autaptic STX1AWT, STX1ALEOpen, STX1ALEOpen + ∆N2-9, STX1ALEOpen + ∆N2-19, or STX1ALEOpen + ∆N2-28 neurons. (E) Example traces (left) and quantification of the charge transfer (right) of sucrose-elicited readily releasable pools (RRPs) obtained from the same neurons as in (D). (F) Quantification of probability of vesicular release (Pvr) determined as the percentage of the RRP released upon one action potential (AP). (G) Example traces (left) and quantification (right) of paired-pulse ratio (PPR) measured at 40 Hz. (H) Example traces (left) and quantification of the frequency (right) of mEPSCs. (I) Quantification of mEPSC rate as spontaneous release of one unit of RRP. (I) Quantification of mEPSC rate as spontaneous release of one unit of RRP.
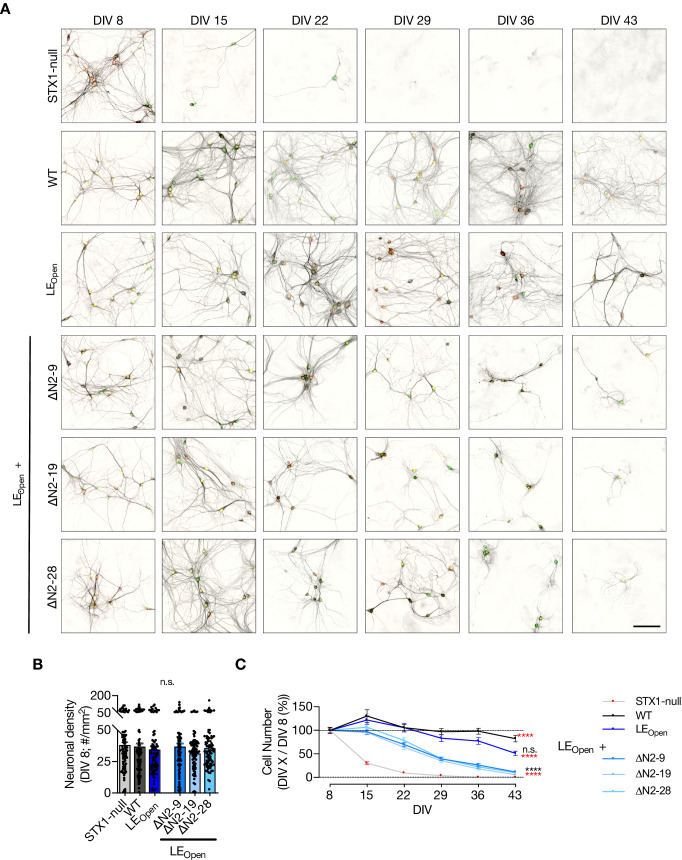
Figure 4—figure supplement 1. Interruption of both Munc18-1 binding modes of STX1 ultimately leads to neuronal death.
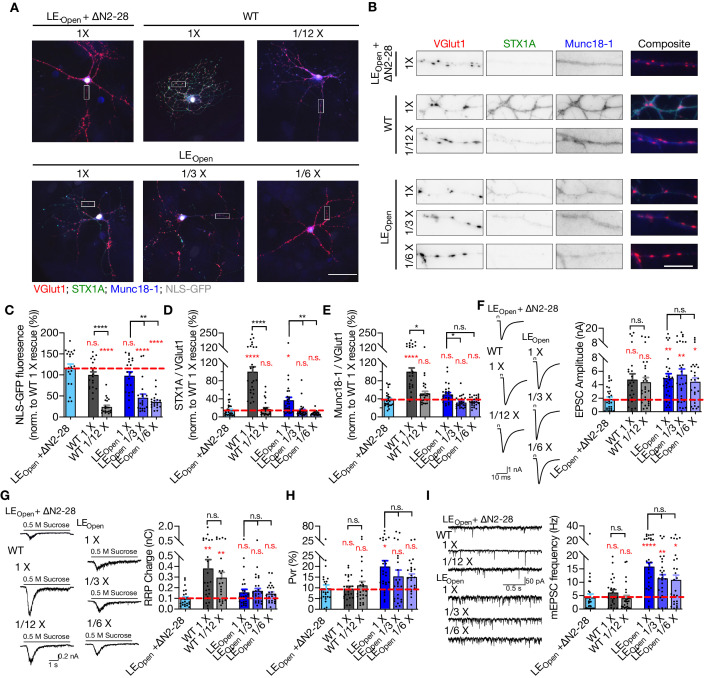
Figure 4—figure supplement 2. Reducing the expression levels of STX1AWT or STX1ALEOpen does not alter their synaptic release properties.
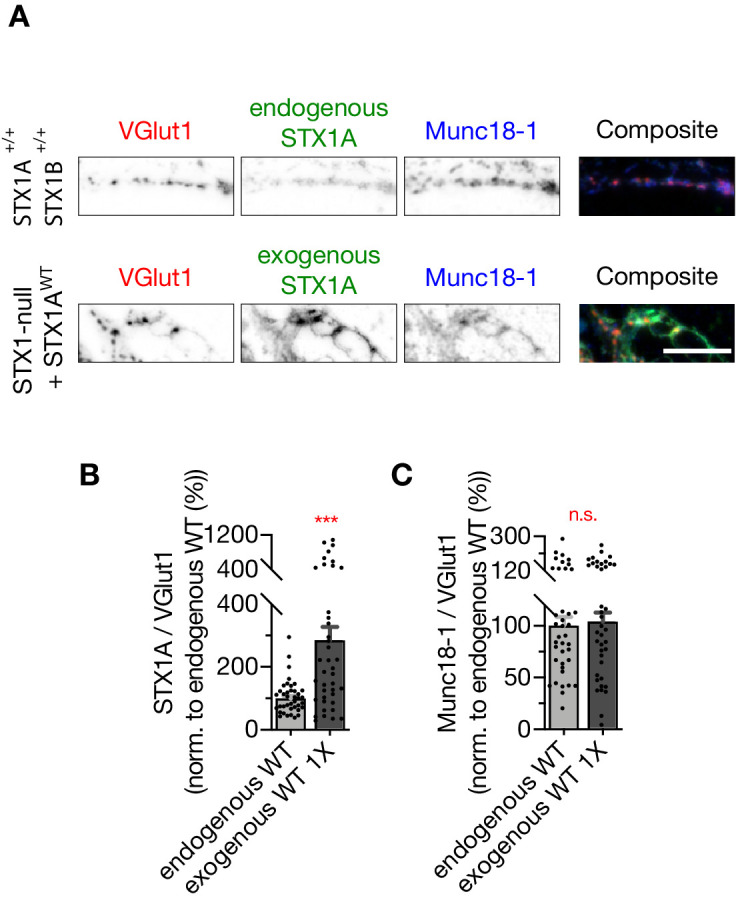
Figure 4—figure supplement 3. Exogenous expression of STX1A using 1× volume of lentiviral particles is approximately threefold higher than endogenous STX1A expression.