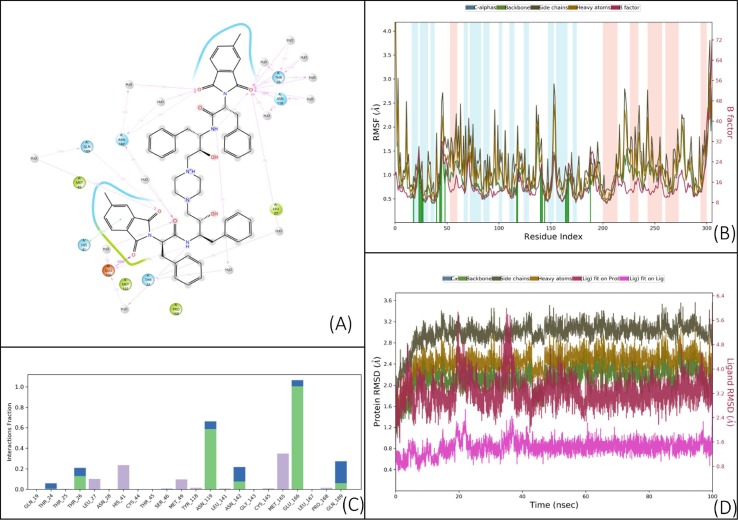
Fig. 5.
Results of a 100 ns (ns) MD simulation of compound V. (A) Schematic 2D representation of bound ligand interactions throughout the simulation. (B) Root mean square fluctuation between the binding site of the target protein and interacting ligand. (C) Critical protein–ligand contacts of amino acid side chain residues with the interaction properties. (D) Root mean square deviations difference between the Main protease (3CLPro) and bound ligand V (<4 Å). The graph was obtained for the RMSF value of ligand (purple line) from the protein backbone (green line). While the ligand was tightly bound to the active site throughout the simulation due to multiple interacting amino acids. The uniform spikes throughout simulation point to the maintenance of strain on both ligand and receptor throughout long simulations. This could be due to strain overpowered by interactions. The large interaction interface does help to map out the binding properties of the target site.