Abstract
Vascular aging is characterized by alterations in the constitutive properties and biological functions of the blood vessel wall. Endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) are indispensability elements in the inner layer and the medial layer of the blood vessel wall, respectively. Dipeptidyl peptidase-4 (DPP4) inhibitors, as a hypoglycemic agent, play a protective role in reversing vascular aging regardless of their effects in meliorating glycemic control in humans and animal models of type 2 diabetes mellitus (T2DM) through complex cellular mechanisms, including improving EC dysfunction, promoting EC proliferation and migration, alleviating EC senescence, obstructing EC apoptosis, suppressing the proliferation and migration of VSMCs, increasing circulating endothelial progenitor cell (EPC) levels, and preventing the infiltration of mononuclear macrophages. All of these showed that DPP4 inhibitors may exert a positive effect against vascular aging, thereby preventing vascular aging-related diseases. In the current review, we will summarize the cellular mechanism of DPP4 inhibitors regulating vascular aging; moreover, we also intend to compile the roles and the promising therapeutic application of DPP4 inhibitors in vascular aging-related diseases.
Keywords: DPP4 inhibitors, vascular aging, diseases, endothelial cells, vascular smooth muscle cells, endothelial progenitor cells, mononuclear macrophages
Introduction
Vascular aging is a complex process characterized by a progressive loss of biological integrity and functionality, which increases mortality with advancing age. Accumulating proof illustrated that vascular aging not only augments the independent risk of cardiovascular diseases, including coronary artery disease (CAD), hypertension, heart failure, and dyslipidemia, but also plays a pivotal role in the etiology of common clinical outcomes, such as neurodegenerative diseases, cerebrovascular diseases, and psychological diseases, implying that vascular aging is a serious threat to human health and life.
Dipeptidyl peptidase-4 (DPP4), also known as cell surface antigen CD26, is an omnipresent transmembrane protease that cleaves NH2-terminal dipeptides from their abundant substrates, such as glucagon-like peptide-1 (GLP-1), glucagon-dependent insulinotropic polypeptide (GIP), stromal cell-derived factor-1α/C-X-C chemokine receptor type-4 (SDF-1α/CXCR4), and vasoactive peptides and neuropeptides, which are responsible for glucose metabolism, inflammation, vascular function, and immunity. DPP4 inhibitors are a novel available antihyperglycemic drug approved for treating type 2 diabetes mellitus (T2DM) in clinical practice, decreasing DPP4 enzyme activity and further increasing the concentration of DPP4 substrates. Numerous studies concluded that DPP4 expression occurs in the vascular system, which includes endothelial cells (ECs) (1), vascular smooth muscle cells (VSMCs) (2), cardiomyocytes (3), mononuclear cells (4), and many other cell types. There is a belief that DPP4 may help prevent cardiovascular disease through multiple mechanisms.
In recent years, a large number of studies have been conducted to indicate the protective roles of DPP4 inhibitors in the vascular system. Emerging evidences also suggested that DPP4 inhibitors play a causative role in improving vascular function and delaying vascular aging. It has been reported that DPP4 inhibitors exert vascular beneficial roles beyond glycemic control, which degrade the risk for further development of serious comorbidities associated with T2DM, especially chronic vascular complications (5). On one hand, DPP4 inhibitors take part in the control of vascular aging by inhibiting inflammation and oxidative stress at the molecular level. On the other hand, DPP4 inhibitors exert pleiotropic effects on cardiovascular diseases directly through complicated cellular mechanisms, containing improving EC dysfunction, promoting EC proliferation and migration, alleviating EC senescence, obstructing EC apoptosis, suppressing the proliferation and migration of VSMCs, increasing circulating endothelial progenitor cell (EPCs) levels, and preventing the infiltration of mononuclear macrophages. As a consequence, new therapeutic agents for vascular aging are needed to prevent vascular aging-related diseases.
Although manifest progress has been made in the understanding of vascular aging pathogenesis, the underlying interaction between DPP4 inhibitors and vascular aging remains unknown. There has been no review on the role of DPP4 inhibitors and mediated mechanisms in vascular aging. Therefore, this review is to compile the cumulative and current study on DPP4 inhibitors regulating vascular aging, thereby providing theoretical guidance and clinical application for reversing vascular aging-related diseases.
The Mechanism of DPP4 Inhibitors in Vascular Aging
Vascular aging, an independent risk factor of cardiovascular diseases and an important element in progression to organ aging, is highly related to alterations in the constitutive properties and biological functions of the blood vessel wall (6). Mechanistically, ECs (Table 1) and VSMCs (Table 2) are involved in the pathogenesis of vascular aging, and structural damage and dysfunction of these cells, such as dysfunction, proliferation, migration, senescence, and apoptosis, are tightly linked to cardiovascular events in humans (26). Indeed, population studies have demonstrated that dysfunction of circulating EPCs (Table 3) and mononuclear macrophages (Table 3) also plays an important role in vascular aging (37, 38). DPP4 inhibitors exert pleiotropic effects on cardiovascular diseases directly through complicated cellular mechanisms as described above. Herein we discussed the potential mechanism of DPP4 inhibitors in vascular aging at cellular levels.
Table 1.
DPP4 inhibitors associated with vascular function and mechanisms in ECs aging.
| Vascular aging | DPP4 inhibitors | Functions | Mechanisms | References |
|---|---|---|---|---|
| ECs dysfunction | vildagliptin | attenuate ECs dysfunction | activateTRPV4 and meditates Ca2+ uptake | (7) |
| linagliptin | ameliorate ECs dysfunction | decrease oxidative stress | (8) | |
| saxagliptin | improve ECs dysfunction | inhibit AP-1 and NF-κB pathway | (9) | |
| anagliptin | against ECs dysfunction | inhibit NLRP3 inflammasome activation | (10) | |
| sitagliptin | inhibit ECs dysfunction | inhibit TNF-α | (11) | |
| sitagliptin | restore ECs dysfunction | activate beta-adrenergic receptor | (12) | |
| linagliptin | restrain ECs dysfunction | prevent the decrease of KL expression | (13) | |
| ECs proliferation and migration | DPP4 inhibitors | increase proliferation and migration of rBMVECs | mediate SIRT1/HIF‐1α/VEGF signaling pathway | (14) |
| teneligliptin | increases HUVECs proliferation | administrate cell-cycle inhibitors hallmarks expression (P27, P21 and P53), and decreasing proapoptotic genes (BAX and CASP3) | (15) | |
| sitagliptin | promote human aortic ECs proliferation | enhance expression of VEGF | (16) | |
| anagliptin | increase of ECs migration | activate SOD-1/RhoA/MAPK) signaling | (17) | |
| ECs senescence | saxagliptin | attenuate ECs senescence | regulate AMPK /SIRT1/ Nrf2 signaling pathway | (18) |
| DPP4 inhibitors | reverse HUVECs senescence | modulate PKA signaling | (19) | |
| ECs apoptosis | sitagliptin | dampen endothelial apoptosis | SDF-1α/CXCR4/Stat3 signaling pathways | (20) |
TRPV4, transient receptor potential vanilloid 4; AP-1, activator protein 1; NF-κB, nuclear factor κB; NLRP3, NLR family, pyrin domain containing 3; TNF-α, tumor necrosis factor alpha; KL, klotho; SIRT1, sirtuin 1; VEGF, vascular endothelial growth factor; SOD-1, superoxide dismutase 1; MAPK, mitogen-activated protein kinase; Nrf2, nuclear factor erythroid 2-related factor 2; PKA, protein kinase A; SDF-1α, stromal cell-derived factor-1α; CXCR4, C-X-C chemokine receptor type-4; Stat3, signal transducer and activator of transcription 3.
Table 2.
DPP4 inhibitors associated with vascular function and mechanisms in VSMCs aging.
| Vascular aging | DPP4 inhibitors | Functions | Mechanisms | References |
|---|---|---|---|---|
| VSMCs proliferation and migration | anagliptin | downregulate the proliferation of VSMCs | restrain ERK phosphorylation | (21) |
| linagliptin | weaken VSMCs proliferation | regulate caspase-3-mediated apoptosis of VSMCs | (22) | |
| gemigliptin | anti-proliferative roles in | activate NRF-2 signaling pathway and inhibit expression of MCP-1 and VCAM-1 | (23) | |
| sitagliptin | ameliorate VSMCs | prevent Akt/MAPK signaling pathway via upregulating PTEN | (24) | |
| vildagliptin | suppress VSMCs proliferation | activate ER stress/NF-κB pathway | (2) | |
| linagliptin | moderate VSMCs proliferation | decrease VSMCs DNA synthesis | (25) |
ERK, extracellular signal-regulated kinase; NRF-2, nuclear factor erythroid 2-related factor 2; MCP-1, monocyte chemoattractant protein-1; VCAM-1, vascular cell adhesion molecule-1; Akt, v-akt murine thymoma viral oncogene homologue; MAPK, mitogen-activated protein kinase; PTEN, phosphatase and tensin homolog deleted on chromosome ten; ER, endoplasmic reticulum; NF-κB, nuclear factor κB.
Table 3.
DPP4 inhibitors associated with vascular function and mechanisms in EPCs and mononuclear macrophage aging.
| Vascular aging | DPP4 inhibitors | Functions | Mechanisms | References |
|---|---|---|---|---|
| EPC aging | Sitagliptin | Protect EPC function | Activate AMPK/ULK1 signaling pathway | (27) |
| Sitagliptin | Promote EPC mobilization | Increase plasma SDF-1 and GLP-1 level | (28) | |
| Saxagliptin | Recruits EPCs from bone marrow | SDF-1α/CXCR4 axis | (29) | |
| Linagliptin | Improve circulating EPC function | Promote CD34/CXCR4 activity | (30) | |
| Sitagliptin | Increase EPC count | SDF-1α/CXCR4 axis | (31, 32) | |
| Mononuclear and macrophage aging | Teneligliptin | Reduce ox-LDL uptake and foam cell formation | Inhibit the expression of CD36 and ACAT-1 gene | (33) |
| Gemigliptin | Inhibit foam cell formation | Akt/AMPK-dependent NF-κB and JNK signaling | (34) | |
| Anagliptin | Suppress TNF-1α-induced monocyte migration | Increase adenosine receptor signal pathway | (21) | |
| Sitagliptin | Recruit circulating monocytes | Upregulate the serum levels of MCP-1 | (32) | |
| Anagliptin | Hinder macrophage accumulation | anti-inflammation | (35) | |
| Trelagliptin | Inhibit monocyte attachment | Inhibit AP-1 and NF-κB signaling | (36) |
MAPK, mitogen-activated protein kinase; ULK1, unc-51-like kinase 1; SDF-1α, stromal cell-derived factor-1α; GLP-1, glucagon-like peptide-1; CXCR4, C-X-C chemokine receptor type-4; ACAT-1, acetyl-CoA acetryltransferase 1; Akt, v-akt murine thymoma viral oncogene homologue; NF-κB, nuclear factor κB; JNK, c-Jun N-terminal kinase; MCP-1, monocyte chemoattractant protein-1; AP-1, activator protein 1.
DPP4 Inhibitors and EC Function
ECs, the inner layer of blood vessels, act as an essential interface between blood and peripheral tissue elements and possess regulating effects on vascular functions including control of vascular permeability, vascular tone, blood coagulation, extracellular matrix remodeling, and inflammatory responses (39, 40). Hyperglycemia is one of the principal factors contributing to the progression of endothelial dysfunction in diabetes mellitus (41). The clinical studies included small numbers of subjects with T2DM, and the results confirmed an improvement in the vascular function by DPP4 inhibitors (42, 43). In this regard, we will discuss the relationship between DPP4 inhibitors and EC functions.
DPP4 Inhibitors and EC Dysfunction
EC dysfunction, also called impaired vascular dilatation, is a pivotal initiation and progression of cardiovascular diseases, bringing about organ damage during aging (44, 45). A growing number of in vivo and in vitro findings suggest that DPP4 inhibitors have a protective effect against EC dysfunction. Gao et al. proved that vildagliptin activates transient receptor potential vanilloid 4 (TRPV4) and then meditates Ca2+ uptake, protecting against hyperglycemia-induced EC dysfunction (7). And vildagliptin attenuated EC dysfunction and shortened atherosclerotic lesions in nondiabetic apolipoprotein E-deficient (ApoE−/−) mice, which means that the effects of vildagliptin are independent of the glucose reducing function (46). Linagliptin suppressed the development atherosclerotic lesions in ApoE−/− mice beyond antiglucose action, which is closely relevant to the amelioration of EC dysfunction through decreasing oxidative stress (8). Sitagliptin reduced inflammation-related EC dysfunction and positively ameliorated diastolic dysfunction (DD) in male Dahl salt-sensitive rats (47). It has been recommended that the DPP4 inhibitor saxagliptin improves EC dysfunction induced by oxidized low-density lipoprotein (ox-LDL) via regulating activator protein 1 (AP-1) and the nuclear factor kappaB (NF-κB) pathway (9). Moreover, another result identified that anagliptin provides protection against vascular dysfunction in ECs triggered by hyperglycemia, and the action is mediated by the inhibition of NLR family, pyrin domain containing 3 (NLRP3) inflammasome activation that relies on the sirtuin 1 (SIRT1)-dependent pathway (10). Surprisingly, Goncalves et al. displayed that sitagliptin has a positive capacity of inhibiting vascular EC dysfunction induced by the pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α) (11). Oliveira et al. also found that sitagliptin restores EC dysfunction by the activation of the beta-adrenergic receptor in vivo and in vitro (12). At last, in a clinical study that recruited 40 patients with CAD and uncontrolled T2DM, sitagliptin significantly alleviated EC dysfunction and inflammatory condition, which exhibits potent effects on preventing vascular-related diseases (48).
Klotho (KL), as a longevity gene, is an anti-aging protein against inflammation. KL methylation is associated with accelerated aging (49, 50). The expression of KL in parathyroid, adipocytes, brain, vascular ECs, and kidney has been previously suggested (51–53). There are papers that reported that KL provides a protective role of restraining EC dysfunction (13, 54). Importantly, reduced circulating KL content is strongly related with vascular aging (55). First of all, the DPP4 inhibitor linagliptin significantly prevented the decreased expression of KL in the vessel wall, eventually blocking EC dysfunction and aortic stiffness in female mice on a Western diet in the long term (13). Then, linagliptin improved not only the progression of brain aging but also other various aging phenotypes in KL knockout mice, and these effects were attributed to an increasing nitric oxide (NO) bioavailability in the cerebral vascular system (56). Indeed, linagliptin ameliorated angiotensin II (Ang II) signaling, which decreased the KL levels (57). Consequently, the KL-mediated vasculo-protective effects of linagliptin are considered to be accounted for by both regulating NO signaling, which is the downstream target of KL, and Ang II signaling, which is the upstream target of KL.
It is widely accepted that the advanced glycation end-product (AGE) receptor, RAGE, named for its ability to bind to AGEs, activates different intracellular signaling pathways. AGE–RAGE interaction signals induced EC dysfunction via four pathways: nicotinamide adenine dinucleotide phosphate (NADPH) oxidase–(reactive oxygen species) ROS, Janus kinase (JAK-2)-signal transducer and activator of transcription 1 (STAT 1), mitogen-activated protein kinase (MAPK)–extracellular signal-regulated kinase (ERK), and phosphoinositide-3-kinase (PI3K)–v-akt murine thymoma viral oncogene homologue (Akt), and the phosphorylated NF-κB accelerated the gene expression of growth factors, proinflammatory cytokines, oxidative stress, and profibrotic cytokines (58–60), all of which promoted the occurrence of aging-related cardiovascular diseases. Interestingly, linagliptin could suppress the AGE–RAGE–evoked oxidative stress. DPP4 inhibitors might be a novel therapeutic target for vascular aging in patients with T2DM by block AGE–RAGE axis (61, 62).
DPP4 Inhibitors and EC Proliferation and Migration
The proliferation of ECs plays a crucial role in accelerating endothelial healing and ameliorating vascular dysfunction (63). According to a previous study, it has been concluded that DPP4 inhibitors offer protection from hypoxia/high glucose induced impairments in the proliferation and migration of rat brain microvascular endothelial cells (rBMVECs), and this action may be mediated by the SIRT1/HIF‐1α/vascular endothelial growth factor (VEGF) signaling pathway (14). Pujadas et al. proved that the DPP4 inhibitor teneligliptin increases human umbilical vein endothelial cell (HUVEC) proliferation exposed to hyperglycemia conditions, administrating cell-cycle inhibitor hallmark expression (P27, P21, and P53) and decreasing proapoptotic genes (BAX and CASP3), while promoting the expression of B-cell lymphoma 2 (BCL2) (15). It has been showed that sitagliptin increases circulating SDF-1α levels (64). Neuhaus et al. reported that SDF-1 promotes human aortic EC proliferation by enhancing the expression of VEGF (16). In animal experiments, the author identified an increased proliferation of ECs via the SDF1-CXCR4 axis (65). The clinical trial with sitagliptin individuals with T2DM, which is powered to assess the capacity of this DPP4 inhibitor on SDF-1, demonstrated that sitagliptin remarkably suppresses SDF-1 degradation (66). A recent study described that anagliptin upregulates superoxide dismutase 1 (SOD-1) expression, which, in turn, negatively modulates oxide production and activates SOD-1/RhoA/MAPK signaling, ultimately promoting EC migration (17).
DPP4 Inhibitors and EC Senescence
Recent data have shown that EC senescence is an outstanding contributor to vascular aging, which subsequently results in vascular aging-related diseases (67, 68). Chen et al. designed an animal trial that revealed that DPP4 inhibitors attenuate vascular aging and EC senescence through regulating oxidative stress by means of the AMP-activated protein kinase (AMPK)/SIRT1/nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway; meanwhile, saxagliptin has been shown to restore vascular aging by decreased senescence associated beta-galactosidase (SA-β-gal) activity and protein expression of p53 and p21. In consistency with in vivo studies, knockdown or inhibition of DPP4 reduced H2O2-induced EC senescence of great significance (18). It is speculated that DPP4 inhibitors may be promising therapeutic targets to lessen EC senescence; thereby, DPP4 inhibitors may be a salutary method to combat vascular aging. Similar results obtained from another animal study performed by Oeseburg et al. showed that DPP4 inhibitors reverse reactive oxygen species-induced HUVEC senescence by modulating protein kinase A (PKA) signaling downstream of the GLP-1 receptor (19).
DPP4 Inhibitors and EC Apoptosis
An increased rate of EC apoptosis is responsible for vascular aging, destruction of vessel integrity, and disruption of the endothelial barrier, which is closely associated with various vascular aging-related diseases (69). To examine the roles of DPP4 in high glucose-induced EC autophagy and apoptosis, Zhao et al. utilized small interfering RNA (siRNA) knockdown and detected DPP4 and AMPK at the protein level (70). The results concluded that DPP4 upregulates adenosine AMPK phosphorylation to promote EC apoptosis and autophagy (70). Presumably, DPP4 inhibitors may become dramatic agents against apoptosis and autophagy of ECs that participated in the aging of the vasculature. Interestingly, the DPP4 inhibitor sitagliptin was found to effectively dampen high glucose-induced endothelial apoptosis via the activation of AMPK phosphorylation in ECs (71). There is also evidence supporting the fact that DPP4 inhibitors perform a significant prevention of the apoptosis in HUVECs under hypoxic states, and SDF-1α/CXCR4/signal transducer and activator of transcription 3 (Stat3) signaling pathways can explain this effect of DPP4 inhibitors (20).
DPP4 Inhibitors and VSMC Function
VSMCs, the medial layer of blood vessels and the predominant structural element of the vessel wall, not only modulate vasoconstriction and vasodilation but also control blood stream and blood pressure. Vascular aging engages a variety of pivotal signals that act in concert to produce VSMC phenotypic changes (72). Additionally, the main changes of VSMC phenotypes occur when they are exposed to diverse stimuli, and the conversion is crucial for VSMC proliferation and migration.
DPP4 Inhibitors and VSMC Proliferation and Migration
The proliferation of VSMCs that resulted from various stimulations is the predominant reason of the vascular proliferative events. It is widely known that vascular aging is accompanied by arterial stiffness and arteriosclerosis. Overwhelming evidence indicated that DPP4 inhibitors directly play a favorable impact against VSMC proliferation. The outcome showed that the migration of VSMCs from the media to intima and their proliferation under the synthetic condition are responsible for arterial stiffness and arteriosclerosis; in addition to this, soluble DPP4 was proved to enhance cultured VSMC proliferation, and anagliptin was shown to downregulate the proliferation through restraining ERK phosphorylation (21); DPP4 significantly activates the MAPK and NF-κB signaling pathway, leading to the induction of inflammation and proliferation of VSMCs in vitro (73). Similarly, the conclusive effect of soluble DPP4 on the proliferation and inflammation of human VSMCs by activating the MAPK and NF-κB signaling involving protease-activated receptor 2 (PAR2) was also presented in another study (74). Subsequently, the observation from a previous animal experiment demonstrated that linagliptin weakens VSMC proliferation after endothelial injury regardless of the glucose control effect, which may be inextricably linked to accessorial caspase-3-mediated apoptosis of VSMCs (22). It has been established that gemigliptin has antiproliferative and antimigratory roles in VSMCs by activating nuclear factor erythroid 2-related factor 2 (NRF-2) signaling pathway and inhibiting the expression of the chemokine monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1) (23). SDF-1α/CXCR4 signaling is a contributor to the occurrences of hypertrophy and proliferation in renal microvascular smooth muscle cells (75). Besides, in cultured human pulmonary arterial smooth muscle cells (PASMCs), the DPP4 inhibitor sitagliptin prevented the Akt/MAPK signaling pathway via upregulating phosphatase and tensin homolog deleted on chromosome ten (PTEN) in a dose-dependent manner, leading to the amelioration of the platelet-derived growth factor (PDGF)-BB-induced VSMC proliferation (24). The study with 4 weeks of vildagliptin in diabetic mice suggested that compared with the control group, the stenosis of injured carotid arteries was markedly reduced, and this consequence was achieved via suppressing the VSMC proliferation by activation of the endoplasmic reticulum (ER) stress/NF-κB pathway (2). Recently, Takahashi et al. also reported that combined treatment with linagliptin and empagliflozin moderates VSMC proliferation significantly by attractively decreasing VSMC DNA synthesis in vitro (25). Taken together, these findings imply that DPP4 inhibitors play a preventative role in the development of the VSMC proliferation procedure against vascular aging.
DPP4 Inhibitors and EPC Levels
EPCs, defined as CD34+ cells originating from the bone marrow, are major cardiovascular disease risk biomarkers responsible for endothelial repair and neo-angiogenesis and play a vital role in repairing damaged vessels due to vascular aging (37, 76).
A series of both preclinical and clinical dada observed that DPP4 inhibitors are vascular-protective by controlling the function and number of EPCs, which shows striking correlation with vascular diseases and vascular risks (77). First, sitagliptin, as an intervention for protecting EPC function through enhancing autophagy, promotes ischemic angiogenesis by activating the AMPK/unc-51-like kinase 1 (ULK1) signaling pathway (27). The findings suggested that sitagliptin promotes EPC mobilization from the bone marrow to circulation and augments the migration of EPCs from circulation to kidney parenchyma for angiogenesis (78). Sitagliptin presents a tremendous effect in EPC mobilization, differentiation, and homing ascribed to the increase in the plasma SDF-1 and GLP-1 level independent of blood glucose (28). A very recent study has explored that sitagliptin increases mobilization of circulating EPCs and differentiation of early EPCs (79). Second, saxagliptin recruits EPCs from the bone marrow by SDF-1α/CXCR4 to modulate vasculogenesis in a renal ischemia/reperfusion (I/R) model in a dose-dependent manner, showing antioxidant and anti-inflammatory properties to healing renal injury (29). A randomized, single-site, placebo-controlled, double-blind, phase 4 clinical trial found that combination therapy with saxagliptin and metformin exposes a coefficient effect, which improves circulating EPC function, eventually meliorating arterial stiffness, renal function, and systolic blood pressure (80). Furthermore, in subjects with T2DM with established chronic kidney disease (CKD) who received linagliptin in combination with metformin and/or insulin, the randomized controlled trial illustrated a statistically significant improvement of CD34+ EPC migratory function by promoting CD34/CXCR4 activity (30).
Numerous papers investigated the effect of DPP4 inhibitors on the EPC number. The circulating EPC level was closely correlated to the endothelial function and could be thought of as a pathogenic hallmark of vascular complications, whose damage may expedite the progression of diabetic vasculopathy (81). At first, data from a 12-month recent randomized, open-label active-treatment-controlled clinical trial showed that vildagliptin aggrandizes the circulating EPC number (82). Second, the beneficial effects of both alogliptin and gliclazide on circulating EPC levels in T2DM under poor glucose control have been confirmed by a randomized trial (83), the mechanism of which was reasonably mediated by a glucose-lowering effect. Moreover, the improved effects of sitagliptin on endothelial function in participates with T2DM are probably due to an accessorial number of circulating EPCs (84). When compared to patients with T2DM treated with glimepiride, the group that received sitagliptin for 12 weeks showed an obvious increase in the circulating EPC count, and the effect was likely mediated by the SDF-1α/CXCR4 axis (31). The same results were obtained from a small and non-randomized report, sitagliptin exerted a mediating role in increasing the accumulation of SDF-1, which binds to its receptor, and CXCR4 stimulates the mobilization of EPCs from the bone marrow (77). It has been discovered that sitagliptin upregulates the EPC number in T2DM patients modulated by the SDF-1α/CXCR4 signaling pathway (32), which is in line with the result obtained by Aso et al. (31) and Fadini et al. (32). At last, a surprising consequence turned out that saxagliptin and metformin increased the number of EPCs, and there was no significant difference between the two groups (85).
DPP4 Inhibitors and Mononuclear Macrophage Function
Emerging evidence suggested that the progression of macrophage phagocytosis of extracellular ox-LDL to transform foam cells is one of the early atherosclerotic properties. In obese T2DM mice and patients, teneligliptin has been shown to conspicuously inhibit the expression of CD36 and acetyl-CoA acetryltransferase 1 (ACAT-1) gene, which participate in reducing ox-LDL uptake and foam cell formation of macrophages (33). More recently, the study conducted by Terasaki et al. confirmed that teneligliptin could suppress foam cell formation of macrophages in T1DM by preventing CD36 and ACAT-1 gene expression partially via receding the detrimental effects of AGEs (86). NLRP3 inflammasome activation and interleukin-1β (IL-1β) release contribute to the formation of foam cells (87), which is a characteristic of atherosclerosis (AS). Consistently, it is supported that ox-LDL results in IL-1β excretion in human macrophages by adding NLRP3 expression (88). Dai et al. found that DPP4 inhibitors repress NLRP3 inflammasome activation, toll-like receptor 4 (TLR4) signal, and IL-1β secretion in human macrophages via declining the activation of protein kinase C (PKC) (89). Foam cell formation requires scavenger receptors (SRs) on macrophages, comprising SRA, CD36, and LOX-1 (90). DPP4 inhibitors regress SRs, CD36, and LOX-1 expression through downregulating PKC activity, which is involved in the formation of foam cells (90). Gemigliptin confers an inhibiting effect on foam cell formation from THP-1 macrophages, and this efficient effect was mediated by attenuating Akt/AMPK-dependent NF-κB and c-Jun N-terminal kinase (JNK) signaling (34). In an animal study, compared with the control or the sodium glucose cotransporter 2 (SGLT2) inhibitor ipragliflozin group, DPP4 inhibitor alogliptin alone or the combination group illustrated further blockage of foam cell formation, ultimately suppressing the development of atherosclerotic plaque substantially (91). The recruitment of monocytes from circulation to the endothelium is a prominent factor in the pathophysiology of the atherosclerotic lesion. An inhibitor of DPP4, anagliptin profoundly suppressed TNF-1α-induced monocyte migration, which resulted from an increasing adenosine receptor signal pathway (21). In ApoE−/− mice, GLP-1 restrained chemokine-induced monocyte migration and macrophage matrix metalloproteinase-9 (MMP-9) levels, which are significant procedures in AS (73). Additionally, DPP4 inhibitors decreased the monocyte number and prevented macrophage infiltration (92). MCP-1 is straightly involved in the recruitment process of circulating monocytes (93). Quite interestingly, in a randomized, placebo-controlled, single-blind, crossover clinical study, sitagliptin reveals anti-atherosclerotic influence through upregulating the serum level of MCP-1 (32). Sitagliptin has been recognized to play an impact on anti-atherosclerosis that is of great significance via promoting the alteration into M2 macrophage in plaque and blocking the lipid content of plaque formation; sitagliptin-mediated restraint of AS builds on this M2 polarization through SDF-1/CXCR4 signaling (94). A DPP4 inhibitor anagliptin can adequately hinder the macrophage accumulation plaque area in coronary arteries due to its anti-inflammatory features (35). Moreover, trelagliptin suppressed the adhesion of monocytes to ECs by inhibiting AP-1 and NF-κB signaling, which mediates the process of inflammation and monocyte attachment, accordingly preventing vascular aging-related diseases (36).
The Role of DPP4 Inhibitors in Vascular Aging-Related Diseases
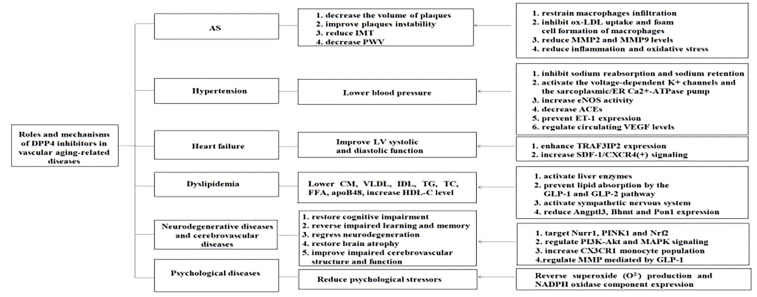
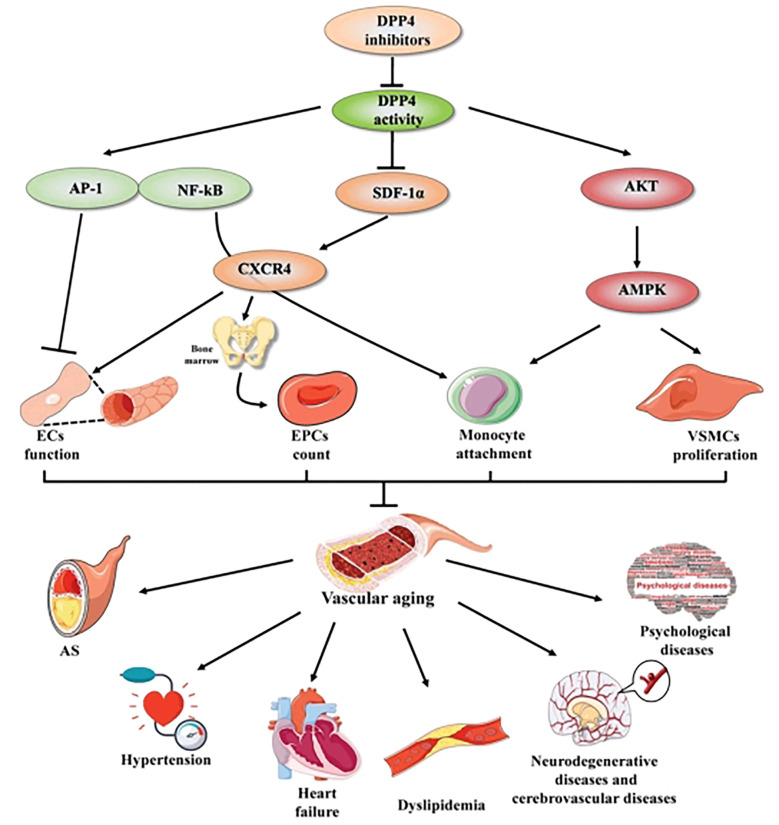
The aging of vasculature is a particular type of organic aging that plays a key role in vascular aging-related diseases. Vascular aging-related diseases are the most common complications and the topmost cause of mortality among older people with T2DM; therefore, addressing aging-related vascular diseases is of great significance. Aging-related functional and structural alterations of the vascular wall contribute to the pathogenesis of vascular aging-related diseases, encompassing AS, hypertension, heart failure, dyslipidemia, neurodegenerative diseases, cerebrovascular diseases, and psychological diseases. Recently, successive studies showed that DPP4 inhibitors play a potent effect against vascular aging-related diseases mentioned above (Figures 1, 2).
Figure 1.
Roles and mechanisms of DPP4 inhibitors in vascular aging -related diseases. DPP4 inhibitors play an effective effect against vascular aging-related diseases through multiple mechanisms. AS, atherosclerosis; JMT, intima-media thickness; PWV, pulse wave velocity; LV, left ventricular; CM, chylomicrons; VLDL, very low density lipoprotein; IDL, intermediate density lipoprotein; TG, triglyceride; TC, total cholesterol; FFA, free fatty acid; apoB48, apolipoprotein B-48; HDL-C, high-density lipoprotein cholesterol; MMP2, macrophage matrix metalloprotcinase-2; MMP9, macrophage matrix metalloproteinasc-9; ER, endoplasmic reticulum; eNOS, endothelial nitric oxide synthase; ACEs, angiotensin-converting enzymes; ET-1, endothelin-1 ; VEGF, vascular endothelial growth factor; TRAF3IP2,TRAF3, Interacting Protein 2; SDF-1, stromal cell-derived factor-Iα; CXCR4, C-X-C chemokine receptor type-4; GLP-1 , glucagon-like peptide-1; GLP-2, glucagon-like peptide-2; Angptl3, angiopoietin-like 3; Bhmt, betaine-homocysteine S-mcthyltransferasc; Pon1, paraoxonasc-1; Nurrl, nuclear receptor related I ; PINK1, PTEN-i nduced putative kinase 1; Nrf2, nuclear factor erythroid 2-related factor 2; PI3K, phosphoinositide-3-kinase; Akt, v-akt murine thymoma viral oncogene homologue; MAPK, mitogen-activated protein kinase; NADPH, nicotinamide adenine dinucleotide phosphate.
Figure 2.
The mechanisms of DPP4 inhibitors in vascular aging and vascular aging-related diseases. DPP4 inhibitors play a beneficial effect in vascular aging not only through multiple cellular mechanisms, including improving ECs function, increasing EPCs count, mediating the process of monocyte attachment and decreasing VSMCs proliferation, but also through complex molecular mechanisms, including AP-I and NF-KB signaling, SDF-1α/CXCR4 axis and Akt/AMPK signaling pathway. Thereby preventing vascular aging-related diseases, compassing AS, hypertension, heart failure, dyslipidemia, neurodegenerative diseases and cerebrovascular diseases, psychological diseases. Arrows indicate stimulatory relationships, indicates inhibitory signal. AP-1, activator protein 1; NF-κB, nuclear factor kappaB; SDF-1, stromal cell- derived factor-1α; CXCR4, C-X-C chemokine receptor type-4; Akt, v-akt murine thymoma viral oncogene homologue; MAPK, mitogen-activated protein kinase; ECs, endothelial cells; VSMCs, vascular smooth muscle cells; EPCs, endothelial progenitor cell; AS, atherosclerosis. Arrows indicate stimulatory relationship, non-arrows indicate inhibitory signal.
DPP4 Inhibitors in AS
AS, as a manifestation of vascular aging and the prominent cause of aging-related cardiovascular disease, is characterized by accumulating lipids and fibrous elements in the vascular wall (95). It is a widespread belief that AS is a complex procedure accompanied by the interaction of diverse cells, lipid, and inflammatory regulators (90). Recently, a growing body of experimental and clinical studies confirmed that DPP4 inhibitors have a favorable role in AS.
In experimental evidences, DPP4 inhibitors substantially restrained macrophage infiltration and markedly decreased the volume of arteriosclerotic plaques in cholesterol-fed rabbits (35). In obese T2DM mice and patients, teneligliptin has been shown to conspicuously inhibit ox-LDL uptake and foam cell formation of macrophages (33). Furthermore, on one hand, sitagliptin negatively modulated the MMP2 and MMP9 levels and reduced plaque MMP9 expression; on the other hand, sitagliptin added amounts of plaque collagen in ApoE knockout mice, which contributes to the stability of arteriosclerotic lesions (73, 92). In addition, combined application with a DPP4 inhibitor and an SGLT2 inhibitor revealed the synergistical suppression of plaque lesion in the aortic root in diabetic mice (91). In consistency with that, teneligliptin inhibited the progression of AS in the aortic arch in ApoE−/− mice that received 20-week drug management by reducing macrophage accumulation, lipid deposition, and MCP-1 expression (96).
DPP4 inhibitors, as anti-atherosclerotic drugs, are being increasingly exploited in clinical research. Firstly, in a prospective, randomized, open-label parallel group trial, DPP4 inhibitors reversed the origination and progression of carotid AS evaluated as intima-media thickness (IMT) in T2DM individuals treated with 12 weeks of sitagliptin and vildagliptin by reducing daily inflammation and oxidative stress. Secondly, a randomized controlled trial, the sitagliptin preventive study of intima-media thickness (IMT) evaluation (SPIKE), demonstrated that sitagliptin has greater reduction in the mean and left maximum IMT in the 104-week treatment group (97). Indeed, the result from a study of preventive effects of alogliptin on diabetic atherosclerosis (SPEAD-A) suggested that alogliptin changes the maximum and mean IMT of carotid arteries, which was estimated by echography (98). In addition, another randomized study analyzed that sitagliptin can regress carotid IMT in patients with T2DM (99), which is consistent with the SPIKE trial. Lastly, aortic pulse wave velocity (PWV) is a hallmark of early AS; decreased PWV was observed in T2DM subjects with 26 weeks of linagliptin treatment (100). However, the PROLOGUE randomized controlled trial concluded that there are no differences on the occurrence of carotid IMT in the sitagliptin administrated group when compared with conventional therapy (101). A further sub-analysis of the PROLOGUE study seems in accordance with this view that sitagliptin has no beneficial effect on the endothelial function and arterial stiffness, which may be conductive to the progression of AS (102–104). Therefore, the roles of DPP4 inhibitors on carotid IMT remain to be investigated. Collectively, DPP4 inhibitors pose potent effects in the management of AS.
DPP4 Inhibitors in Hypertension
Hypertension is an increasing condition with multiple risk factors, and the occurrence of hypertension is enormous among elderly individuals. Vascular aging, such as arterial stiffness and AS, may be responsible for hypertension in the aging population (105).
A large amount of studies have showed that DPP4 inhibitors are capable of reducing blood pressure levels. A randomized, double-blind, placebo-controlled, three-period, crossover study supported that sitagliptin has a modest antihypertensive function in nondiabetic patients (106). Likewise, it has been documented that sitagliptin has a small reduction in 24-ambulatory blood pressure (BP), which is independent of the glycemic control and body mass index (BMI) (107). Nevertheless, some trials observed that sitagliptin reduces BP significantly (107, 108). In addition, data from a double-blind, randomized, controlled study enrolling 2,000 previously drug-free subjects with T2DM who treated with the drug once or twice daily at a dose of 50 mg for 24 weeks showed that vildagliptin obviously lowered both systolic and diastolic BP (109). Susanne et al. found that using empagliflozin and linagliptin together substantially improved central BP and vascular function compared to a combination of metformin and insulin (110).
The underlying mechanisms for the roles of DPP4 inhibitors on BP are complicated and obscure. First, DPP4 inhibitors enhance the activity of GLP-1, which has been proved to inhibit sodium reabsorption from the proximal tubules and decrease sodium retention, presenting a beneficial impact on BP (111). Second, vildagliptin induced vasorelaxation through the activation of voltage-dependent K+ channels and the sarcoplasmic/ER Ca2+-ATPase pump. Third, inhibition of DPP4 increases endothelial nitric oxide synthase (eNOS) activity, causing the NO release and resulting in the dilatation of the vascular wall (112). Fourth, the vascular dilatation induced by acetylcholine and sodium nitroprusside seems to be attributed to the decreased expression of vasoconstrictor-related enzymes afforded by linagliptin, including angiotensin-converting enzymes (ACEs) (113). Additionally, in diabetic rats, sitagliptin prevented the expression of endothelin-1 (ET-1) in the aortic endothelium via inhibiting the NF-κB/inhibitor of the NF-κB system by activation of the AMPK pathway, which produces a vascular protection function against hypertension (114). Lastly, vildagliptin performed an antihypertensive action via regulating circulating VEGF levels in patients with diabetes and hypertension (115).
DPP4 Inhibitors in Heart Failure
Vascular aging is a main factor that leads to arterial stiffness, which is responsible for changes in afterload and left ventricular (LV) geometry (116). Numerous lines of proofs from experimental and clinical studies have showed that the favorable properties of DPP4 inhibitors on the progression of heart failure connected with poorer cardiovascular outcomes (117–119), especially DD (120, 121). Alogliptin ameliorated coronary flow reserve (CFR) and left ventricular election fraction (LVEF) assessed by magnetic resonance imaging (MRI) in subjects with T2DM and CAD (122). The same result was achieved by McCormick et al. who found that sitagliptin, another DPP4 inhibitor, improves LV dysfunction in patients with T2DM and CAD during dobutamine stress and a reduction in post-ischemic stunning (123). An animal study showed that use of linagliptin results in the improvement in DD in two rodent models of insulin resistance and obesity. In one study, linagliptin ameliorated DD in insulin-resistant 8-week-old Zucker obese (ZO) rats (124). In another study, linagliptin reversed the development of DD in western diet (WD)−fed mice via enhancing the expression of TRAF3 Interacting Protein 2 (TRAF3IP2) and inhibiting oxidant stress and fibrosis (13, 57, 120). DPP4 inhibitors decreased the ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity (E/e’) and increased the ratio of peak early to late diastolic filling velocity (E/A), respectively, in patients with T2DM after acute myocardial infarction (AMI), indicating a beneficial effect on LV diastolic failure (125). The inhibition of DPP4 meliorated cardiac function after AMI by increased SDF-1, which promoted the accumulation of CXCR4(+) bone marrow-derived stem cells into the ischemic area (126, 127). Some studies found that DPP4 inhibitors possess a significant improvement not only in the LV systolic function but also in the diastolic function. For example, patients from a pilot study treated with 12 months of DPP4 inhibitors had an obvious amelioration in systolic, diastolic, and endothelial function (128). In contrast, current evidence from clinical studies has shown that DPP4 inhibitors have no effects on myocardial function in subjects with T2DM and heart failure (129, 130). Data from the EXAMINE trial showed that alogliptin failed to increase the incidence of heart failure outcomes compared with placebo in participants with T2DM after an acute coronary syndrome (131, 132). Same results were obtained from the TECOS trial; sitagliptin has no clinical effect on cardiovascular outcomes (133). Consistently, linagliptin did not increase the risk of heart failure-related outcomes, including among patients with and without a history of heart failure (134). However, some studies proved that DPP4 inhibitors increase the risk of hospital admission for heart failure in those patients with existing risk factors of cardiovascular system or cardiovascular diseases when compared with the control group (135). In SAVOR-TIMI53, saxagliptin was associated with a 27% increased risk of heart failure hospitalization, particularly in the first 12 months (136); the complex mechanisms and potential effects for this have not been fully understood. In consequence, the mechanisms and clinical significance of DPP4 inhibitors on the heart warrant further investigation.
DPP4 Inhibitors in Dyslipidemia
Existing evidence demonstrated that lipid profiles are highly linked to increased arterial stiffness, which eventually causes vascular aging. Particularly, triglyceride (TG)/high-density lipoprotein cholesterol (HDL-C) ratio, known as the atherogenic index, is a predictor for early vascular aging (137). Population trials have suggested that various DPP4 inhibitors dramatically decrease the level of low-density lipoprotein (LDL) cholesterol, TG, total cholesterol (TC), and free fatty acid (FFA) and add the level of HDL-C both in animal and patient models (138–141). Alogliptin has been showed that it is equipped to downregulate lipids, thereby delaying AS (142). Fukuda-Tsuru et al. found that both single and repeated treatment of teneligliptin lower the level of TG and FFA in plasma under nonfasting states (143). Interestingly, vildagliptin presented a beneficial fasting lipid particle that was related to slight weight loss (109). In participants treated with vildagliptin/metformin, they revealed significantly lower TC and TG and substantially higher HDL-C levels when compared with the glimepiride/metformin treated group (144). Vildagliptin decreased TG and apolipoprotein B-48 (apoB48) in patients treated with a fat rich meal (145). Similarly, evidence from a multicenter, randomized study confirmed that use of anagliptin results in a reduction in the level of fasting apoB48, which is the prominent apolipoprotein of chylomicrons (CM), very low density lipoprotein (VLDL), intermediate density lipoprotein (IDL), and LDL profiles, and the latent mechanism may be attributed to the suppression of the intestinal lipid transport (146). Anagliptin significantly attenuated TC and LDL-C levels, as well as ameliorated glycemic control, especially in female patients (147). Furthermore, sitagliptin downregulated postprandial levels of apoB48-containing lipoproteins, plasma TG, and FFA (148). Observation from a prospective, randomized, multicenter study found that sitagliptin decreased lipid plaque volume in the coronary artery, which was assessed by integrated backscatter (IB)-intravascular ultrasound (IVUS); a marked decrease in the non-HDL cholesterol level may explain this phenomenon (149).
The underlying mechanisms of DPP4 inhibitors in dyslipidemia remain exclusive. First, DPP4 inhibitors lower the secretion of intestinal TG-rich lipoproteins and transform the activation of liver enzymes, which take part in the synthesis and oxidation of lipid (139). Second, DPP4 inhibitors prevent lipid absorption by the GLP-1 and GLP-2 pathway (150). Moreover, DPP4 inhibitors promote postprandial lipid oxidation and mobilization via the activation of the sympathetic nervous system (151). Postprandial hypertriglyceridemia exerts a significant role in EC dysfunction and accelerates the development of AS (152). To elaborate the molecular mechanism of lipid lowering impact, the study performed by Zhang et al. found that vildagliptin decreased circulating TC and reversed EC dysfunction in the aorta of diabetic rats that benefited from the reduced expression of angiopoietin-like 3 (Angptl3) and betaine-homocysteine S-methyltransferase (Bhmt) and the elevated activation of paraoxonase-1 (Pon1) (138). Clearly, DPP4 inhibitors are considered to be advantageous for individuals with diabetes and dyslipidemia.
DPP4 Inhibitors in Neurodegenerative Diseases and Cerebrovascular Diseases
Parkinson’s disease (PD) and Alzheimer’s disease (AD) are age-related neurodegenerative diseases. DPP4 inhibitors are probably novel approaches to the treatment of PD and cognitive impairment by targeting specific pathophysiology proteins, such as nuclear receptor related 1 (Nurr1), PTEN-induced putative kinase 1 (PINK1), and Nrf2 (153). DPP4 inhibitors reversed the impaired learning and memory and regressed AD-like neurodegeneration by GLP-1 signal pathway inclusion of PI3K-Akt and MAPK (154). Recently, saxagliptin revealed a neuroprotective effect on AD, and this effect depends on its functions of anti-inflammatory, antioxidant, antiapoptotic, and neuroprotective mechanisms (155). Linagliptin presented neuroprotective properties in T2DM patients with neurodegenerative disorders through increasing CX3CR1 monocyte population (156). In a diabetic db/db mice model, linagliptin has also been suggested to restore impaired cognitive function and brain atrophy caused by transient cerebral ischemia (157). There is also evidence that that DPP4 inhibitors, mainly including linagliptin, appear to have a potentially favorable impact on improving impaired cerebrovascular structure and function (158, 159). One of the possibly detailed mechanisms is the regulation of MMP mediated by GLP-1 (160, 161). Hence, the DPP4 inhibitor is a conceivable consideration for therapy of neurodegenerative diseases and cerebrovascular diseases.
DPP4 Inhibitors in Psychological Diseases
Psychological stress can be thought of as a risk factor for vascular aging and vascular aging-related diseases, and oxidative stress plays an important role in vascular senescence in humans and animals (162). Data from the INTERHEART study that recruited 11,119 cases and 13,648 controls from 51 countries illustrated that chronic psychological stressors including depression and anxiety, perception of stress, low sense of control, and life events increase the occurrence of acute myocardial infarction noticeably (163). Indeed, it has been well documented that chronic psychological stressors can increase the likelihood of cardiovascular diseases, hypertension, diabetes mellitus, and metabolic syndrome (164–166). However, some trials demonstrated that diverse stress is harmful to plasma and tissue DPP4 levels (167). Specifically, DPP4 inhibition by anagliptin not only increased the infiltration of macrophage and the expressions of inflammatory molecules, reversing vascular aging and AS, but also reversed superoxide production and NADPH oxidase component expression in ApoE knockout mice (167). Thereby, DPP4 inhibitors can be considered as potential targets against psychological diseases, delaying vascular aging under stress conditions.
Conclusions
The alternations in the physiological integrity and functionality of blood vessel wall contribute to the aging of vasculature, eventually causing vascular aging-related diseases. DPP4 inhibitors, as a novel antihyperglycemic drug, can efficaciously reduce blood glucose levels. In addition, DPP4 inhibitors also exert pleiotropic impacts in delaying vascular aging beyond glycemic control. The potential mechanisms in this regard at cellular levels are complicated, including improving EC dysfunction, promoting EC proliferation and migration, alleviating EC senescence, obstructing EC apoptosis, increasing circulating EPC levels, suppressing the proliferation and migration of VSMCs, and preventing the infiltration of mononuclear macrophages, all of which showed that DPP4 inhibitors may exert a positive effect against vascular aging, thereby preventing vascular aging-related diseases. In conclusion, DPP4 inhibitors are promising therapeutic targets in the administration of vascular aging and vascular aging-related diseases. However, the safety and efficacy of DPP4 inhibitors have not been fully explored; the mechanisms and clinical significance of DPP4 inhibitors on preventing and treating vascular aging and vascular aging-related diseases warrant further investigation, and further experimental and clinical trial is required.
Author Contributions
FC wrote the manuscript. KW and Y-ZZ collected the literature, drew the figures, and supervised the manuscript. Z-WB conceived the idea and had been involved in manuscript conception and drafting. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Kawasaki T, Chen W, Htwe YM, Tatsumi K, Dudek SM. DPP4 Inhibition by Sitagliptin Attenuates LPS-Induced Lung Injury in Mice. Am J Physiol Lung Cell Mol Physiol (2018) 315(5):L834–l45. 10.1152/ajplung.00031.2018 [DOI] [PubMed] [Google Scholar]
- 2.Ji Y, Ge Y, Xu X, Ye S, Fan Y, Zhang J, et al. Vildagliptin Reduces Stenosis of Injured Carotid Artery in Diabetic Mouse Through Inhibiting Vascular Smooth Muscle Cell Proliferation via ER Stress/NF-kappaB Pathway. Front Pharmacol (2019) 10:142. 10.3389/fphar.2019.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnbaum Y, Tran D, Bajaj M, Ye Y. DPP-4 Inhibition by Linagliptin Prevents Cardiac Dysfunction and Inflammation by Targeting the Nlrp3/ASC Inflammasome. Basic Res Cardiol (2019) 114(5):35. 10.1007/s00395-019-0743-0 [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Jimenez D, Petrillo MG, Busada JT, Hermoso MA, Cidlowski JA. Glucocorticoids Mobilize Macrophages by Transcriptionally Up-Regulating the Exopeptidase DPP4. J Biol Chem (2020) 295(10):3213–27. 10.1074/jbc.RA119.010894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akoumianakis I, Antoniades C. Dipeptidyl Peptidase IV Inhibitors as Novel Regulators of Vascular Disease. Vascul Pharmacol (2017) 96-98:1–4. 10.1016/j.vph.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Thijssen DH, Carter SE, Green DJ. Arterial Structure and Function in Vascular Ageing: Are You as Old as Your Arteries? J Physiol (2016) 594(8):2275–84. 10.1113/JP270597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao P, Li L, Wei X, Wang M, Hong Y, Wu H, et al. Activation of Transient Receptor Potential Channel Vanilloid 4 by DPP-4 (Dipeptidyl Peptidase-4) Inhibitor Vildagliptin Protects Against Diabetic Endothelial Dysfunction. Hypertension (2020) 75(1):150–62. 10.1161/HYPERTENSIONAHA.119.13778 [DOI] [PubMed] [Google Scholar]
- 8.Salim HM, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Yagi S, et al. Dipeptidyl Peptidase-4 Inhibitor, Linagliptin, Ameliorates Endothelial Dysfunction and Atherogenesis in Normoglycemic Apolipoprotein-E Deficient Mice. Vascul Pharmacol (2016) 79:16–23. 10.1016/j.vph.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 9.Ma S, Bai Z, Wu H, Wang W. The DPP-4 Inhibitor Saxagliptin Ameliorates Ox-LDL-Induced Endothelial Dysfunction by Regulating AP-1 and NF-KappaB. Eur J Pharmacol (2019) 851:186–93. 10.1016/j.ejphar.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 10.Jiang T, Jiang D, Zhang L, Ding M, Zhou H. Anagliptin Ameliorates High Glucose-Induced Endothelial Dysfunction via Suppression of NLRP3 Inflammasome Activation Mediated by SIRT1. Mol Immunol (2019) 107:54–60. 10.1016/j.molimm.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 11.Goncalves A, Almeida L, Silva AP, Fontes-Ribeiro C, Ambrosio AF, Cristovao A, et al. The Dipeptidyl Peptidase-4 (DPP-4) Inhibitor Sitagliptin Ameliorates Retinal Endothelial Cell Dysfunction Triggered by Inflammation. BioMed Pharmacother (2018) 102:833–8. 10.1016/j.biopha.2018.03.144 [DOI] [PubMed] [Google Scholar]
- 12.Oliveira BC, Marques VB, Brun BF, de Oliveira ESHM, Freitas Soares Melo S, Oliveira EM, et al. Dipeptidyl Peptidase-4 Inhibition Prevents Vascular Dysfunction Induced by Beta-Adrenergic Hyperactivity. BioMed Pharmacother (2019) 113:108733. 10.1016/j.biopha.2019.108733 [DOI] [PubMed] [Google Scholar]
- 13.Manrique C, Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, et al. Dipeptidyl Peptidase-4 Inhibition With Linagliptin Prevents Western Diet-Induced Vascular Abnormalities in Female Mice. Cardiovasc Diabetol (2016) 15:94. 10.1186/s12933-016-0414-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mi DH, Fang HJ, Zheng GH, Liang XH, Ding YR, Liu X, et al. DPP-4 Inhibitors Promote Proliferation and Migration of Rat Brain Microvascular Endothelial Cells Under Hypoxic/High-Glucose Conditions, Potentially Through the SIRT1/HIF-1/VEGF Pathway. CNS Neurosci Ther (2019) 25(3):323–32. 10.1111/cns.13042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pujadas G, De Nigris V, Prattichizzo F, La Sala L, Testa R, Ceriello A. The Dipeptidyl Peptidase-4 (DPP-4) Inhibitor Teneligliptin Functions as Antioxidant on Human Endothelial Cells Exposed to Chronic Hyperglycemia and Metabolic High-Glucose Memory. Endocrine (2017) 56(3):509–20. 10.1007/s12020-016-1052-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuhaus T, Stier S, Totzke G, Gruenewald E, Fronhoffs S, Sachinidis A, et al. Stromal Cell-Derived Factor 1alpha (SDF-1alpha) Induces Gene-Expression of Early Growth Response-1 (Egr-1) and VEGF in Human Arterial Endothelial Cells and Enhances VEGF Induced Cell Proliferation. Cell proliferation (2003) 36(2):75–86. 10.1046/j.1365-2184.2003.00262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Zhang M, Xuan L, Liu Y, Chen C. Anagliptin Inhibits Neointimal Hyperplasia After Balloon Injury via Endothelial Cell-Specific Modulation of SOD-1/RhoA/JNK Signaling in the Arterial Wall. Free Radic Biol Med (2018) 121:105–16. 10.1016/j.freeradbiomed.2018.04.580 [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Yu J, Fu M, Dong R, Yang Y, Luo J, et al. Dipeptidyl Peptidase-4 Inhibition Improves Endothelial Senescence by Activating AMPK/SIRT1/Nrf2 Signaling Pathway. Biochem Pharmacol (2020) 177:113951. 10.1016/j.bcp.2020.113951 [DOI] [PubMed] [Google Scholar]
- 19.Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Sillje HH. Glucagon-Like Peptide 1 Prevents Reactive Oxygen Species-Induced Endothelial Cell Senescence Through the Activation of Protein Kinase A. Arterioscler Thromb Vasc Biol (2010) 30(7):1407–14. 10.1161/ATVBAHA.110.206425 [DOI] [PubMed] [Google Scholar]
- 20.Nagamine A, Hasegawa H, Hashimoto N, Yamada-Inagawa T, Hirose M, Kobara Y, et al. The Effects of DPP-4 Inhibitor on Hypoxia-Induced Apoptosis in Human Umbilical Vein Endothelial Cells. J Pharmacol Sci (2017) 133(1):42–8. 10.1016/j.jphs.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 21.Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S, et al. Anagliptin, a DPP-4 Inhibitor, Suppresses Proliferation of Vascular Smooth Muscles and Monocyte Inflammatory Reaction and Attenuates Atherosclerosis in Male Apo E-Deficient Mice. Endocrinology (2013) 154(3):1260–70. 10.1210/en.2012-1855 [DOI] [PubMed] [Google Scholar]
- 22.Terawaki Y, Nomiyama T, Kawanami T, Hamaguchi Y, Takahashi H, Tanaka T, et al. Dipeptidyl Peptidase-4 Inhibitor Linagliptin Attenuates Neointima Formation After Vascular Injury. Cardiovasc Diabetol (2014) 13:154. 10.1186/s12933-014-0154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi SH, Park S, Oh CJ, Leem J, Park KG, Lee IK. Dipeptidyl Peptidase-4 Inhibition by Gemigliptin Prevents Abnormal Vascular Remodeling via NF-E2-Related Factor 2 Activation. Vascul Pharmacol (2015) 73:11–9. 10.1016/j.vph.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Wang J, He M, Han H, Xie W, Wang H, et al. Dipeptidyl Peptidase IV (DPP-4) Inhibition Alleviates Pulmonary Arterial Remodeling in Experimental Pulmonary Hypertension. Lab Invest (2018) 98(10):1333–46. 10.1038/s41374-018-0080-1 [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Nomiyama T, Terawaki Y, Horikawa T, Kawanami T, Hamaguchi Y, et al. Combined Treatment With DPP-4 Inhibitor Linagliptin and SGLT2 Inhibitor Empagliflozin Attenuates Neointima Formation After Vascular Injury in Diabetic Mice. Biochem Biophys Rep (2019) 18:100640. 10.1016/j.bbrep.2019.100640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-Mediated Dilation and Cardiovascular Risk Prediction: A Systematic Review With Meta-Analysis. Int J Cardiol (2013) 168(1):344–51. 10.1016/j.ijcard.2012.09.047 [DOI] [PubMed] [Google Scholar]
- 27.Dai X, Zeng J, Yan X, Lin Q, Wang K, Chen J, et al. Sitagliptin-Mediated Preservation of Endothelial Progenitor Cell Function via Augmenting Autophagy Enhances Ischaemic Angiogenesis in Diabetes. J Cell Mol Med (2018) 22(1):89–100. 10.1111/jcmm.13296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CY, Shih CM, Tsao NW, Lin YW, Huang PH, Wu SC, et al. Dipeptidyl Peptidase-4 Inhibitor Improves Neovascularization by Increasing Circulating Endothelial Progenitor Cells. Br J Pharmacol (2012) 167(7):1506–19. 10.1111/j.1476-5381.2012.02102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamel NM, Abd El Fattah MA, El-Abhar HS, Abdallah DM. Novel Repair Mechanisms in a Renal Ischaemia/Reperfusion Model: Subsequent Saxagliptin Treatment Modulates the Pro-Angiogenic GLP-1/cAMP/VEGF, ANP/eNOS/NO, SDF-1alpha/CXCR4, and Kim-1/STAT3/HIF-1alpha/VEGF/eNOS Pathways. Eur J Pharmacol (2019) 861:172620. 10.1016/j.ejphar.2019.172620 [DOI] [PubMed] [Google Scholar]
- 30.Awal HB, Nandula SR, Domingues CC, Dore FJ, Kundu N, Brichacek B, et al. Linagliptin, When Compared to Placebo, Improves CD34+ve Endothelial Progenitor Cells in Type 2 Diabetes Subjects With Chronic Kidney Disease Taking Metformin and/or Insulin: A Randomized Controlled Trial. Cardiovasc Diabetol (2020) 19(1):72. 10.1186/s12933-020-01046-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aso Y, Jojima T, Iijima T, Suzuki K, Terasawa T, Fukushima M, et al. Sitagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Increases the Number of Circulating CD34(+)CXCR4(+) Cells in Patients With Type 2 Diabetes. Endocrine (2015) 50(3):659–64. 10.1007/s12020-015-0688-5 [DOI] [PubMed] [Google Scholar]
- 32.Fadini GP, Bonora BM, Cappellari R, Menegazzo L, Vedovato M, Iori E, et al. Acute Effects of Linagliptin on Progenitor Cells, Monocyte Phenotypes, and Soluble Mediators in Type 2 Diabetes. J Clin Endocrinol Metab (2016) 101(2):748–56. 10.1210/jc.2015-3716 [DOI] [PubMed] [Google Scholar]
- 33.Terasaki M, Hiromura M, Mori Y, Kohashi K, Kushima H, Koshibu M, et al. A Dipeptidyl Peptidase-4 Inhibitor Suppresses Macrophage Foam Cell Formation in Diabetic Db/Db Mice and Type 2 Diabetes Patients. Int J Endocrinol (2018) 2018:8458304. 10.1155/2018/8458304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang HJ, Chung HS, Jung TW, Ryu JY, Hong HC, Seo JA, et al. The Dipeptidyl Peptidase-IV Inhibitor Inhibits the Expression of Vascular Adhesion Molecules and Inflammatory Cytokines in HUVECs via Akt- and AMPK-Dependent Mechanisms. Mol Cell Endocrinol (2015) 405:25–34. 10.1016/j.mce.2015.01.025 [DOI] [PubMed] [Google Scholar]
- 35.Hirano T, Yamashita S, Takahashi M, Hashimoto H, Mori Y, Goto M. Anagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Decreases Macrophage Infiltration and Suppresses Atherosclerosis in Aortic and Coronary Arteries in Cholesterol-Fed Rabbits. Metabolism (2016) 65(6):893–903. 10.1016/j.metabol.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 36.Meng J, Zhang W, Wang C, Xiong S, Wang Q, Li H, et al. The Dipeptidyl Peptidase (DPP)-4 Inhibitor Trelagliptin Inhibits IL-1beta-Induced Endothelial Inflammation and Monocytes Attachment. Int Immunopharmacol (2020) 89(Pt B):106996. 10.1016/j.intimp.2020.106996 [DOI] [PubMed] [Google Scholar]
- 37.Balistreri CR, Pisano C, Bertoldo F, Massoud R, Dolci S, Ruvolo G. Red Blood Cell Distribution Width, Vascular Aging Biomarkers, and Endothelial Progenitor Cells for Predicting Vascular Aging and Diagnosing/Prognosing Age-Related Degenerative Arterial Diseases. Rejuvenation Res (2019) 22(5):399–408. 10.1089/rej.2018.2144 [DOI] [PubMed] [Google Scholar]
- 38.Byrne AJ, Powell JE, O'Sullivan BJ, Ogger PP, Hoffland A, Cook J, et al. Dynamics of Human Monocytes and Airway Macrophages During Healthy Aging and After Transplant. J Exp Med (2020) 217(3):e20191236. 10.1084/jem.20191236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aroor AR, Demarco VG, Jia G, Sun Z, Nistala R, Meininger GA, et al. The Role of Tissue Renin-Angiotensin-Aldosterone System in the Development of Endothelial Dysfunction and Arterial Stiffness. Front Endocrinol (Lausanne) (2013) 4:161. 10.3389/fendo.2013.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potenza MA, Nacci C, De Salvia MA, Sgarra L, Collino M, Montagnani M. Targeting Endothelial Metaflammation to Counteract Diabesity Cardiovascular Risk: Current and Perspective Therapeutic Options. Pharmacol Res (2017) 120:226–41. 10.1016/j.phrs.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 41.Singh A, Friden V, Dasgupta I, Foster RR, Welsh GI, Tooke JE, et al. High Glucose Causes Dysfunction of the Human Glomerular Endothelial Glycocalyx. Am J Physiol Renal Physiol (2011) 300(1):F40–8. 10.1152/ajprenal.00103.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin Lowers Albuminuria on Top of Recommended Standard Treatment in Patients With Type 2 Diabetes and Renal Dysfunction. Diabetes Care (2013) 36(11):3460–8. 10.2337/dc13-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ott C, Kistner I, Keller M, Friedrich S, Willam C, Bramlage P, et al. Effects of Linagliptin on Renal Endothelial Function in Patients With Type 2 Diabetes: A Randomised Clinical Trial. Diabetologia (2016) 59(12):2579–87. 10.1007/s00125-016-4083-4 [DOI] [PubMed] [Google Scholar]
- 44.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, et al. Endothelial Dysfunction and Angiogenesis Impairment in the Ageing Vasculature. Nat Rev Cardiol (2018) 15(9):555–65. 10.1038/s41569-018-0030-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial Dysfunction as a Target for Prevention of Cardiovascular Disease. Diabetes Care (2009) 32 Suppl 2:S314–21. 10.2337/dc09-S330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aini K, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Yagi S, et al. Vildagliptin, a DPP-4 Inhibitor, Attenuates Endothelial Dysfunction and Atherogenesis in Nondiabetic Apolipoprotein E-Deficient Mice. Int Heart J (2019) 60(6):1421–9. 10.1536/ihj.19-117 [DOI] [PubMed] [Google Scholar]
- 47.Esposito G, Cappetta D, Russo R, Rivellino A, Ciuffreda LP, Roviezzo F, et al. Sitagliptin Reduces Inflammation, Fibrosis and Preserves Diastolic Function in a Rat Model of Heart Failure With Preserved Ejection Fraction. Br J Pharmacol (2017) 174(22):4070–86. 10.1111/bph.13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, et al. Dipeptidyl Peptidase-4 Inhibitor, Sitagliptin, Improves Endothelial Dysfunction in Association With Its Anti-Inflammatory Effects in Patients With Coronary Artery Disease and Uncontrolled Diabetes. Circ J (2013) 77(5):1337–44. 10.1253/circj.cj-12-1168 [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, Sun Z. Molecular Basis of Klotho: From Gene to Function in Aging. Endocr Rev (2015) 36(2):174–93. 10.1210/er.2013-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolf EJ, Logue MW, Zhao X, Daskalakis NP, Morrison FG, Escarfulleri S, et al. PTSD and the Klotho Longevity Gene: Evaluation of Longitudinal Effects on Inflammation via DNA Methylation. Psychoneuroendocrinology (2020) 117:104656. 10.1016/j.psyneuen.2020.104656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim SC, Liu JJ, Subramaniam T, Sum CF. Elevated Circulating Alpha-Klotho by Angiotensin II Receptor Blocker Losartan Is Associated With Reduction of Albuminuria in Type 2 Diabetic Patients. J Renin Angiotensin Aldosterone Syst (2014) 15(4):487–90. 10.1177/1470320313475905 [DOI] [PubMed] [Google Scholar]
- 52.Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, et al. Alpha-Klotho Expression in Human Tissues. J Clin Endocrinol Metab (2015) 100(10):E1308–18. 10.1210/jc.2015-1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donate-Correa J, Mora-Fernandez C, Martinez-Sanz R, Muros-de-Fuentes M, Perez H, Meneses-Perez B, et al. Expression of FGF23/KLOTHO System in Human Vascular Tissue. Int J Cardiol (2013) 165(1):179–83. 10.1016/j.ijcard.2011.08.850 [DOI] [PubMed] [Google Scholar]
- 54.Kuwahara N, Sasaki S, Kobara M, Nakata T, Tatsumi T, Irie H, et al. HMG-CoA Reductase Inhibition Improves Anti-Aging Klotho Protein Expression and Arteriosclerosis in Rats With Chronic Inhibition of Nitric Oxide Synthesis. Int J Cardiol (2008) 123(2):84–90. 10.1016/j.ijcard.2007.02.029 [DOI] [PubMed] [Google Scholar]
- 55.Castellano G, Intini A, Stasi A, Divella C, Gigante M, Pontrelli P, et al. Complement Modulation of Anti-Aging Factor Klotho in Ischemia/Reperfusion Injury and Delayed Graft Function. Am J Transplant (2016) 16(1):325–33. 10.1111/ajt.13415 [DOI] [PubMed] [Google Scholar]
- 56.Hasegawa Y, Hayashi K, Takemoto Y, Cheng C, Takane K, Lin B, et al. DPP-4 Inhibition With Linagliptin Ameliorates the Progression of Premature Aging in Klotho-/- Mice. Cardiovasc Diabetol (2017) 16(1):154. 10.1186/s12933-017-0639-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aroor AR, Habibi J, Kandikattu HK, Garro-Kacher M, Barron B, Chen D, et al. Dipeptidyl Peptidase-4 (DPP-4) Inhibition With Linagliptin Reduces Western Diet-Induced Myocardial TRAF3IP2 Expression, Inflammation and Fibrosis in Female Mice. Cardiovasc Diabetol (2017) 16(1):61. 10.1186/s12933-017-0544-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of Advanced Glycation End Products in Cellular Signaling. Redox Biol (2014) 2:411–29. 10.1016/j.redox.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Senatus LM, Schmidt AM. The AGE-RAGE Axis: Implications for Age-Associated Arterial Diseases. Front Genet (2017) 8:187. 10.3389/fgene.2017.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanajou D, Ghorbani Haghjo A, Argani H, Aslani S. AGE-RAGE Axis Blockade in Diabetic Nephropathy: Current Status and Future Directions. Eur J Pharmacol (2018) 833:158–64. 10.1016/j.ejphar.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 61.Nakashima S, Matsui T, Takeuchi M, Yamagishi SI. Linagliptin Blocks Renal Damage in Type 1 Diabetic Rats by Suppressing Advanced Glycation End Products-Receptor Axis. Horm Metab Res (2014) 46(10):717–21. 10.1055/s-0034-1371892 [DOI] [PubMed] [Google Scholar]
- 62.Kaifu K, Ueda S, Nakamura N, Matsui T, Yamada-Obara N, Ando R, et al. Advanced Glycation End Products Evoke Inflammatory Reactions in Proximal Tubular Cells via Autocrine Production of Dipeptidyl Peptidase-4. Microvascular Res (2018) 120:90–3. 10.1016/j.mvr.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 63.Lin X, Zhan JK, Wang YJ, Tan P, Chen YY, Deng HQ, et al. Function, Role, and Clinical Application of MicroRNAs in Vascular Aging. BioMed Res Int (2016) 2016:6021394. 10.1155/2016/6021394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papazafiropoulou AK, Papanas N, Trikkalinou A, Fousteris E, Melidonis A. The Oral Dipeptidyl-Peptidase-4 Inhibitor Sitagliptin Increases Circulating Levels Of Stromal-Derived Factor-1 Alpha. Exp Clin Endocrinol Diabetes (2018) 126(6):367–70. 10.1055/s-0043-118748 [DOI] [PubMed] [Google Scholar]
- 65.Remm F, Krankel N, Lener D, Drucker DJ, Sopper S, Brenner C. Sitagliptin Accelerates Endothelial Regeneration After Vascular Injury Independent From GLP1 Receptor Signaling. Stem Cells Int (2018) 2018:5284963. 10.1155/2018/5284963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lovshin JA, Rajasekeran H, Lytvyn Y, Lovblom LE, Khan S, Alemu R, et al. Dipeptidyl Peptidase 4 Inhibition Stimulates Distal Tubular Natriuresis and Increases in Circulating SDF-1alpha(1-67) in Patients With Type 2 Diabetes. Diabetes Care (2017) 40(8):1073–81. 10.2337/dc17-0061 [DOI] [PubMed] [Google Scholar]
- 67.Yang D, Xiao C, Long F, Wu W, Huang M, Qu L, et al. Fra-1 Plays a Critical Role in Angiotensin II-Induced Vascular Senescence. FASEB J (2019) 33(6):7603–14. 10.1096/fj.201801671RRRR [DOI] [PubMed] [Google Scholar]
- 68.Donato AJ, Machin DR, Lesniewski LA. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ Res (2018) 123(7):825–48. 10.1161/CIRCRESAHA.118.312563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Li D, Xu Y, Zhang Y, Tao L, Li S, et al. Essential Oils From Fructus A. Zerumbet Protect Human Aortic Endothelial Cells From Apoptosis Induced by Ox-LDL In Vitro. Evid Based Complement Alternat Med (2014) 2014:956824. 10.1155/2014/956824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao X, Su L, He X, Zhao B, Miao J. Long Noncoding RNA CA7-4 Promotes Autophagy and Apoptosis via Sponging MIR877-3P and MIR5680 in High Glucose-Induced Vascular Endothelial Cells. Autophagy (2020) 16(1):70–85. 10.1080/15548627.2019.1598750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu C, Hu S, Wang N, Tian J. Dipeptidyl Peptidase4 Inhibitor Sitagliptin Prevents High Glucoseinduced Apoptosis via Activation of AMPactivated Protein Kinase in Endothelial Cells. Mol Med Rep (2017) 15(6):4346–51. 10.3892/mmr.2017.6501 [DOI] [PubMed] [Google Scholar]
- 72.Gomez D, Owens GK. Smooth Muscle Cell Phenotypic Switching in Atherosclerosis. Cardiovasc Res (2012) 95(2):156–64. 10.1093/cvr/cvs115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim S, Choi SH, Shin H, Cho BJ, Park HS, Ahn BY, et al. Effect of a Dipeptidyl Peptidase-IV Inhibitor, Des-Fluoro-Sitagliptin, on Neointimal Formation After Balloon Injury in Rats. PloS One (2012) 7(4):e35007. 10.1371/journal.pone.0035007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wronkowitz N, Gorgens SW, Romacho T, Villalobos LA, Sanchez-Ferrer CF, Peiro C, et al. Soluble DPP4 Induces Inflammation and Proliferation of Human Smooth Muscle Cells via Protease-Activated Receptor 2. Biochim Biophys Acta (2014) 1842(9):1613–21. 10.1016/j.bbadis.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 75.Jackson EK, Zhang Y, Gillespie DD, Zhu X, Cheng D, Jackson TC. SDF-1alpha (Stromal Cell-Derived Factor 1alpha) Induces Cardiac Fibroblasts, Renal Microvascular Smooth Muscle Cells, and Glomerular Mesangial Cells to Proliferate, Cause Hypertrophy, and Produce Collagen. J Am Heart Assoc (2017) 6(11):e007253. 10.1161/JAHA.117.007253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turgeon J, Haddad P, Dussault S, Groleau J, Maingrette F, Perez G, et al. Protection Against Vascular Aging in Nox2-Deficient Mice: Impact on Endothelial Progenitor Cells and Reparative Neovascularization. Atherosclerosis (2012) 223(1):122–9. 10.1016/j.atherosclerosis.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 77.Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, et al. The Oral Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Increases Circulating Endothelial Progenitor Cells in Patients With Type 2 Diabetes: Possible Role of Stromal-Derived Factor-1alpha. Diabetes Care (2010) 33(7):1607–9. 10.2337/dc10-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sung PH, Chen KH, Li YC, Chiang JY, Lee MS, Yip HK. Sitagliptin and Shock Wave-Supported Peripheral Blood Derived Endothelial Progenitor Cell Therapy Effectively Preserves Residual Renal Function in Chronic Kidney Disease in Rat-Role of Dipeptidyl Peptidase 4 Inhibition. BioMed Pharmacother (2019) 111:1088–102. 10.1016/j.biopha.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 79.Lin FY, Shih CM, Huang CY, Tsai YT, Loh SH, Li CY, et al. Dipeptidyl Peptidase-4 Inhibitor Decreases Allograft Vasculopathy Via Regulating the Functions of Endothelial Progenitor Cells in Normoglycemic Rats. Cardiovasc Drugs Ther (2020). 10.1007/s10557-020-07013-w [DOI] [PubMed] [Google Scholar]
- 80.Dore FJ, Domingues CC, Ahmadi N, Kundu N, Kropotova Y, Houston S, et al. The Synergistic Effects of Saxagliptin and Metformin on CD34+ Endothelial Progenitor Cells in Early Type 2 Diabetes Patients: A Randomized Clinical Trial. Cardiovasc Diabetol (2018) 17(1):65. 10.1186/s12933-018-0709-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liao YF, Chen LL, Zeng TS, Li YM, Fan Y, Hu LJ, et al. Number of Circulating Endothelial Progenitor Cells as a Marker of Vascular Endothelial Function for Type 2 Diabetes. Vasc Med (2010) 15(4):279–85. 10.1177/1358863X10367537 [DOI] [PubMed] [Google Scholar]
- 82.Dei Cas A, Spigoni V, Cito M, Aldigeri R, Ridolfi V, Marchesi E, et al. Vildagliptin, But Not Glibenclamide, Increases Circulating Endothelial Progenitor Cell Number: A 12-Month Randomized Controlled Trial in Patients With Type 2 Diabetes. Cardiovasc Diabetol (2017) 16(1):27. 10.1186/s12933-017-0503-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Negro R, Greco EL, Greco G. Alogliptin and Gliclazide Similarly Increase Circulating Endothelial Progenitor Cells in Type 2 Diabetes Patients. Exp Clin Endocrinol Diabetes (2019) 127(4):215–9. 10.1055/s-0043-122383 [DOI] [PubMed] [Google Scholar]
- 84.Nakamura K, Oe H, Kihara H, Shimada K, Fukuda S, Watanabe K, et al. DPP-4 Inhibitor and Alpha-Glucosidase Inhibitor Equally Improve Endothelial Function in Patients With Type 2 Diabetes: EDGE Study. Cardiovasc Diabetol (2014) 13:110. 10.1186/s12933-014-0110-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li F, Chen J, Leng F, Lu Z, Ling Y. Effect of Saxagliptin on Circulating Endothelial Progenitor Cells and Endothelial Function in Newly Diagnosed Type 2 Diabetic Patients. Exp Clin Endocrinol Diabetes (2017) 125(6):400–7. 10.1055/s-0042-124421 [DOI] [PubMed] [Google Scholar]
- 86.Terasaki M, Yashima H, Mori Y, Saito T, Matsui T, Hiromura M, et al. A Dipeptidyl Peptidase-4 Inhibitor Inhibits Foam Cell Formation of Macrophages in Type 1 Diabetes via Suppression of CD36 and ACAT-1 Expression. Int J Mol Sci (2020) 21(13):4811. 10.3390/ijms21134811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Satoh T, Kambe N, Matsue H. NLRP3 Activation Induces ASC-Dependent Programmed Necrotic Cell Death, Which Leads to Neutrophilic Inflammation. Cell Death Dis (2013) 4:e644. 10.1038/cddis.2013.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu W, Yin Y, Zhou Z, He M, Dai Y. OxLDL-Induced IL-1 Beta Secretion Promoting Foam Cells Formation was Mainly via CD36 Mediated ROS Production Leading to NLRP3 Inflammasome Activation. Inflammation Res (2014) 63(1):33–43. 10.1007/s00011-013-0667-3 [DOI] [PubMed] [Google Scholar]
- 89.Dai Y, Dai D, Wang X, Ding Z, Mehta JL. DPP-4 Inhibitors Repress NLRP3 Inflammasome and Interleukin-1beta via GLP-1 Receptor in Macrophages Through Protein Kinase C Pathway. Cardiovasc Drugs Ther (2014) 28(5):425–32. 10.1007/s10557-014-6539-4 [DOI] [PubMed] [Google Scholar]
- 90.Dai Y, Wang X, Ding Z, Dai D, Mehta JL. DPP-4 Inhibitors Repress Foam Cell Formation by Inhibiting Scavenger Receptors Through Protein Kinase C Pathway. Acta Diabetol (2014) 51(3):471–8. 10.1007/s00592-013-0541-3 [DOI] [PubMed] [Google Scholar]
- 91.Terasaki M, Hiromura M, Mori Y, Kohashi K, Kushima H, Ohara M, et al. Combination Therapy With a Sodium-Glucose Cotransporter 2 Inhibitor and a Dipeptidyl Peptidase-4 Inhibitor Additively Suppresses Macrophage Foam Cell Formation and Atherosclerosis in Diabetic Mice. Int J Endocrinol (2017) 2017:1365209. 10.1155/2017/1365209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vittone F, Liberman A, Vasic D, Ostertag R, Esser M, Walcher D, et al. Sitagliptin Reduces Plaque Macrophage Content and Stabilises Arteriosclerotic Lesions in Apoe (-/-) Mice. Diabetologia (2012) 55(8):2267–75. 10.1007/s00125-012-2582-5 [DOI] [PubMed] [Google Scholar]
- 93.Fenyo IM, Gafencu AV. The Involvement of the Monocytes/Macrophages in Chronic Inflammation Associated With Atherosclerosis. Immunobiology (2013) 218(11):1376–84. 10.1016/j.imbio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 94.Brenner C, Franz WM, Kuhlenthal S, Kuschnerus K, Remm F, Gross L, et al. DPP-4 Inhibition Ameliorates Atherosclerosis by Priming Monocytes Into M2 Macrophages. Int J Cardiol (2015) 199:163–9. 10.1016/j.ijcard.2015.07.044 [DOI] [PubMed] [Google Scholar]
- 95.Palombo C, Kozakova M. Arterial Stiffness, Atherosclerosis and Cardiovascular Risk: Pathophysiologic Mechanisms and Emerging Clinical Indications. Vascul Pharmacol (2016) 77:1–7. 10.1016/j.vph.2015.11.083 [DOI] [PubMed] [Google Scholar]
- 96.Salim HM, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Yagi S, et al. Teneligliptin, a Dipeptidyl Peptidase-4 Inhibitor, Attenuated Pro-Inflammatory Phenotype of Perivascular Adipose Tissue and Inhibited Atherogenesis in Normoglycemic Apolipoprotein-E-Deficient Mice. Vascul Pharmacol (2017) 96-98:19–25. 10.1016/j.vph.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 97.Katakami N, Mita T, Irie Y, Takahara M, Matsuoka TA, Gosho M, et al. Effect of Sitagliptin on Tissue Characteristics of the Carotid Wall in Patients With Type 2 Diabetes: A Post Hoc Sub-Analysis of the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE). Cardiovasc Diabetol (2018) 17(1):24. 10.1186/s12933-018-0666-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, et al. Alogliptin, a Dipeptidyl Peptidase 4 Inhibitor, Prevents the Progression of Carotid Atherosclerosis in Patients With Type 2 Diabetes: The Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A). Diabetes Care (2016) 39(1):139–48. 10.2337/dc15-0781 [DOI] [PubMed] [Google Scholar]
- 99.Mita T, Katakami N, Shiraiwa T, Yoshii H, Gosho M, Shimomura I, et al. The Effect of Sitagliptin on the Regression of Carotid Intima-Media Thickening in Patients With Type 2 Diabetes Mellitus: A Post Hoc Analysis of the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation. Int J Endocrinol (2017) 2017:1925305. 10.1155/2017/1925305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Boer SA, Heerspink HJL, Juárez Orozco LE, van Roon AM, Kamphuisen PW, Smit AJ, et al. Effect of Linagliptin on Pulse Wave Velocity in Early Type 2 Diabetes: A Randomized, Double-Blind, Controlled 26-Week Trial (RELEASE). Diabetes Obes Metab (2017) 19(8):1147–54. 10.1111/dom.12925 [DOI] [PubMed] [Google Scholar]
- 101.Oyama J, Murohara T, Kitakaze M, Ishizu T, Sato Y, Kitagawa K, et al. The Effect of Sitagliptin on Carotid Artery Atherosclerosis in Type 2 Diabetes: The PROLOGUE Randomized Controlled Trial. PloS Med (2016) 13(6):e1002051. 10.1371/journal.pmed.1002051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maruhashi T, Higashi Y, Kihara Y, Yamada H, Sata M, Ueda S, et al. Long-Term Effect of Sitagliptin on Endothelial Function in Type 2 Diabetes: A Sub-Analysis of the PROLOGUE Study. Cardiovasc Diabetol (2016) 15(1):134. 10.1186/s12933-016-0438-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tomiyama H, Miwa T, Kan K, Matsuhisa M, Kamiya H, Nanasato M, et al. Impact of Glycemic Control With Sitagliptin on the 2-Year Progression of Arterial Stiffness: A Sub-Analysis of the PROLOGUE Study. Cardiovasc Diabetol (2016) 15(1):150. 10.1186/s12933-016-0472-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanaka A, Yoshida H, Nanasato M, Oyama JI, Ishizu T, Ajioka M, et al. Sitagliptin on Carotid Intima-Media Thickness in Type 2 Diabetes Patients Receiving Primary or Secondary Prevention of Cardiovascular Disease: A Subgroup Analysis of the PROLOGUE Study. Int J Cardiol (2018) 271:331–5. 10.1016/j.ijcard.2018.05.055 [DOI] [PubMed] [Google Scholar]
- 105.Cheng HM, Park S, Huang Q, Hoshide S, Wang JG, Kario K, et al. Vascular Aging and Hypertension: Implications for the Clinical Application of Central Blood Pressure. Int J Cardiol (2017) 230:209–13. 10.1016/j.ijcard.2016.12.170 [DOI] [PubMed] [Google Scholar]
- 106.Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, et al. Effect of Sitagliptin, a Dipeptidyl Peptidase-4 Inhibitor, on Blood Pressure in Nondiabetic Patients With Mild to Moderate Hypertension. J Clin Pharmacol (2008) 48(5):592–8. 10.1177/0091270008316885 [DOI] [PubMed] [Google Scholar]
- 107.Ogawa S, Ishiki M, Nako K, Okamura M, Senda M, Mori T, et al. Sitagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Decreases Systolic Blood Pressure in Japanese Hypertensive Patients With Type 2 Diabetes. Tohoku J Exp Med (2011) 223(2):133–5. 10.1620/tjem.223.133 [DOI] [PubMed] [Google Scholar]
- 108.Nakamura T, Iwanaga Y, Miyaji Y, Nohara R, Ishimura T, Miyazaki S, et al. Cardiovascular Efficacy of Sitagliptin in Patients With Diabetes at High Risk of Cardiovascular Disease: A 12-Month Follow-Up. Cardiovasc Diabetol (2016) 15:54. 10.1186/s12933-016-0371-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Evans M, Schweizer A, Foley JE. Blood Pressure and Fasting Lipid Changes After 24 Weeks' Treatment With Vildagliptin: A Pooled Analysis in >2,000 Previously Drug-Naive Patients With Type 2 Diabetes Mellitus. Vasc Health Risk Manag (2016) 12:337–40. 10.2147/VHRM.S112148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jung S, Bosch A, Kannenkeril D, Karg MV, Striepe K, Bramlage P, et al. Combination of Empagliflozin and Linagliptin Improves Blood Pressure and Vascular Function in Type 2 Diabetes. Eur Heart J Cardiovasc Pharmacother (2020) 6(6):364–71. 10.1093/ehjcvp/pvz078 [DOI] [PubMed] [Google Scholar]
- 111.Gutzwiller J-P, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, et al. Glucagon-Like Peptide-1 Is Involved in Sodium and Water Homeostasis in Humans. Digestion (2006) 73(2-3):142–50. 10.1159/000094334 [DOI] [PubMed] [Google Scholar]
- 112.Mason RP, Jacob RF, Kubant R, Ciszewski A, Corbalan JJ, Malinski T. Dipeptidyl Peptidase-4 Inhibition With Saxagliptin Enhanced Nitric Oxide Release and Reduced Blood Pressure and sICAM-1 Levels in Hypertensive Rats. J Cardiovasc Pharmacol (2012) 60(5):467–73. 10.1097/FJC.0b013e31826be204 [DOI] [PubMed] [Google Scholar]
- 113.Koibuchi N, Hasegawa Y, Katayama T, Toyama K, Uekawa K, Sueta D, et al. DPP-4 Inhibitor Linagliptin Ameliorates Cardiovascular Injury in Salt-Sensitive Hypertensive Rats Independently of Blood Glucose and Blood Pressure. Cardiovasc Diabetol (2014) 13:157. 10.1186/s12933-014-0157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang S-T, Su H, Zhang QIU, Tang H-Q, Wang C-J, Zhou Q, et al. Sitagliptin Inhibits Endothelin-1 Expression in the Aortic Endothelium of Rats With Streptozotocin-Induced Diabetes by Suppressing the Nuclear Factor-κb/Iκbα System Through the Activation of AMP-Activated Protein Kinase. Int J Mol Med (2016) 37(6):1558–66. 10.3892/ijmm.2016.2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Naggar AR, Zaafar D, Elyamany M, Hassanin S, Bassyouni A, Abdel-Latif H. The Role of Vildagliptin in Treating Hypertension Through Modulating Serum VEGF in Diabetic Hypertensive Patients. J Cardiovasc Pharmacol Ther (2019) 24(3):254–61. 10.1177/1074248418817345 [DOI] [PubMed] [Google Scholar]
- 116.Triposkiadis F, Xanthopoulos A, Butler J. Cardiovascular Aging and Heart Failure: JACC Review Topic of the Week. J Am Coll Cardiol (2019) 74(6):804–13. 10.1016/j.jacc.2019.06.053 [DOI] [PubMed] [Google Scholar]
- 117.Kim YG, Yoon D, Park S, Han SJ, Kim DJ, Lee KW, et al. Dipeptidyl Peptidase-4 Inhibitors and Risk of Heart Failure in Patients With Type 2 Diabetes Mellitus: A Population-Based Cohort Study. Circ Heart Fail (2017) 10(9):e003957. 10.1161/CIRCHEARTFAILURE.117.003957 [DOI] [PubMed] [Google Scholar]
- 118.Wang MT, Lin SC, Tang PL, Hung WT, Cheng CC, Yang JS, et al. The Impact of DPP-4 Inhibitors on Long-Term Survival Among Diabetic Patients After First Acute Myocardial Infarction. Cardiovasc Diabetol (2017) 16(1):89. 10.1186/s12933-017-0572-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.dos Santos L, Salles TA, Arruda-Junior DF, Campos LC, Pereira AC, Barreto AL, et al. Circulating Dipeptidyl Peptidase IV Activity Correlates With Cardiac Dysfunction in Human and Experimental Heart Failure. Circ Heart Fail (2013) 6(5):1029–38. 10.1161/CIRCHEARTFAILURE.112.000057 [DOI] [PubMed] [Google Scholar]
- 120.Bostick B, Habibi J, Ma L, Aroor A, Rehmer N, Hayden MR, et al. Dipeptidyl Peptidase Inhibition Prevents Diastolic Dysfunction and Reduces Myocardial Fibrosis in a Mouse Model of Western Diet Induced Obesity. Metabolism (2014) 63(8):1000–11. 10.1016/j.metabol.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brown SM, Smith CE, Meuth AI, Khan M, Aroor AR, Cleeton HM, et al. Dipeptidyl Peptidase-4 Inhibition With Saxagliptin Ameliorates Angiotensin II-Induced Cardiac Diastolic Dysfunction in Male Mice. Endocrinology (2017) 158(10):3592–604. 10.1210/en.2017-00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kato S, Fukui K, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, et al. Inhibition of DPP-4 by Alogliptin Improves Coronary Flow Reserve and Left Ventricular Systolic Function Evaluated by Phase Contrast Cine Magnetic Resonance Imaging in Patients With Type 2 Diabetes and Coronary Artery Disease. Int J Cardiol (2016) 223:770–5. 10.1016/j.ijcard.2016.08.306 [DOI] [PubMed] [Google Scholar]
- 123.McCormick LM, Kydd AC, Read PA, Ring LS, Bond SJ, Hoole SP, et al. Chronic Dipeptidyl Peptidase-4 Inhibition With Sitagliptin Is Associated With Sustained Protection Against Ischemic Left Ventricular Dysfunction in a Pilot Study of Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease. Circ Cardiovasc Imaging (2014) 7(2):274–81. 10.1161/CIRCIMAGING.113.000785 [DOI] [PubMed] [Google Scholar]
- 124.Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, et al. Dipeptidylpeptidase Inhibition Is Associated With Improvement in Blood Pressure and Diastolic Function in Insulin-Resistant Male Zucker Obese Rats. Endocrinology (2013) 154(7):2501–13. 10.1210/en.2013-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fujiwara T, Yoshida M, Nakamura T, Sakakura K, Wada H, Arao K, et al. Dipeptidyl Peptidase-4 Inhibitors Are Associated With Improved Left Ventricular Diastolic Function After Acute Myocardial Infarction in Diabetic Patients. Heart Vessels (2015) 30(5):696–701. 10.1007/s00380-014-0509-4 [DOI] [PubMed] [Google Scholar]
- 126.Connelly KA, Advani A, Zhang Y, Advani SL, Kabir G, Abadeh A, et al. Dipeptidyl Peptidase-4 Inhibition Improves Cardiac Function in Experimental Myocardial Infarction: Role of Stromal Cell-Derived Factor-1alpha. J Diabetes (2016) 8(1):63–75. 10.1111/1753-0407.12258 [DOI] [PubMed] [Google Scholar]
- 127.Huber BC, Brunner S, Segeth A, Nathan P, Fischer R, Zaruba MM, et al. Parathyroid Hormone Is a DPP-IV Inhibitor and Increases SDF-1-Driven Homing of CXCR4(+) Stem Cells Into the Ischaemic Heart. Cardiovasc Res (2011) 90(3):529–37. 10.1093/cvr/cvr014 [DOI] [PubMed] [Google Scholar]
- 128.Leung M, Leung DY, Wong VW. Effects of Dipeptidyl Peptidase-4 Inhibitors on Cardiac and Endothelial Function in Type 2 Diabetes Mellitus: A Pilot Study. Diabetes Vasc Dis Res (2016) 13(3):236–43. 10.1177/1479164116629352 [DOI] [PubMed] [Google Scholar]
- 129.Brenner C, Adrion C, Grabmaier U, Theisen D, von Ziegler F, Leber A, et al. Sitagliptin Plus Granulocyte Colony-Stimulating Factor in Patients Suffering From Acute Myocardial Infarction: A Double-Blind, Randomized Placebo-Controlled Trial of Efficacy and Safety (SITAGRAMI Trial). Int J Cardiol (2016) 205:23–30. 10.1016/j.ijcard.2015.11.180 [DOI] [PubMed] [Google Scholar]
- 130.McMurray JJV, Ponikowski P, Bolli GB, Lukashevich V, Kozlovski P, Kothny W, et al. Effects of Vildagliptin on Ventricular Function in Patients With Type 2 Diabetes Mellitus and Heart Failure: A Randomized Placebo-Controlled Trial. JACC Heart Fail (2018) 6(1):8–17. 10.1016/j.jchf.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 131.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin After Acute Coronary Syndrome in Patients With Type 2 Diabetes. N Engl J Med (2013) 369(14):1327–35. 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- 132.Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, et al. Heart Failure and Mortality Outcomes in Patients With Type 2 Diabetes Taking Alogliptin Versus Placebo in EXAMINE: A Multicentre, Randomised, Double-Blind Trial. Lancet (London England) (2015) 385(9982):2067–76. 10.1016/s0140-6736(14)62225-x [DOI] [PubMed] [Google Scholar]
- 133.Cornel JH, Bakris GL, Stevens SR, Alvarsson M, Bax WA, Chuang LM, et al. Effect of Sitagliptin on Kidney Function and Respective Cardiovascular Outcomes in Type 2 Diabetes: Outcomes From TECOS. Diabetes Care (2016) 39(12):2304–10. 10.2337/dc16-1415 [DOI] [PubMed] [Google Scholar]
- 134.McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, et al. Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA. Circulation (2019) 139(3):351–61. 10.1161/circulationaha.118.038352 [DOI] [PubMed] [Google Scholar]
- 135.Li L, Li S, Deng K, Liu J, Vandvik PO, Zhao P, et al. Dipeptidyl Peptidase-4 Inhibitors and Risk of Heart Failure in Type 2 Diabetes: Systematic Review and Meta-Analysis of Randomised and Observational Studies. BMJ (2016) 352:i610. 10.1136/bmj.i610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus. N Engl J Med (2013) 369(14):1317–26. 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 137.KiliÇ A, Baydar O, ElÇİk D, Apaydin Z, Can MM. Role of Dyslipidemia in Early Vascular Aging Syndrome. Turkish J Med Sci (2020) 51(2):727–34. 10.3906/sag-2008-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping F, et al. A Possible Mechanism: Vildagliptin Prevents Aortic Dysfunction Through Paraoxonase and Angiopoietin-Like 3. BioMed Res Int (2018) 2018:3109251. 10.1155/2018/3109251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cha SA, Park YM, Yun JS, Lim TS, Song KH, Yoo KD, et al. A Comparison of Effects of DPP-4 Inhibitor and SGLT2 Inhibitor on Lipid Profile in Patients With Type 2 Diabetes. Lipids Health Dis (2017) 16(1):58. 10.1186/s12944-017-0443-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kusunoki M, Sato D, Nakamura T, Oshida Y, Tsutsui H, Natsume Y, et al. The Beneficial Effects of the DPP-4 Inhibitor Alogliptin on Hemoglobin A1c and Serum Lipids in Japanese Patients With Type 2 Diabetes. Drug Res (Stuttg) (2016) 66(1):18–22. 10.1055/s-0035-1547254 [DOI] [PubMed] [Google Scholar]
- 141.Kusunoki M, Sato D, Nakamura T, Oshida Y, Tsutsui H, Natsume Y, et al. DPP-4 Inhibitor Teneligliptin Improves Insulin Resistance and Serum Lipid Profile in Japanese Patients With Type 2 Diabetes. Drug Res (Stuttg) (2015) 65(10):532–4. 10.1055/s-0034-1390419 [DOI] [PubMed] [Google Scholar]
- 142.Kutoh E, Kaneoka N, Hirate M. Alogliptin: A New Dipeptidyl Peptidase-4 Inhibitor With Potential Anti-Atherogenic Properties. Endocr Res (2015) 40(2):88–96. 10.3109/07435800.2014.952743 [DOI] [PubMed] [Google Scholar]
- 143.Fukuda-Tsuru S, Anabuki J, Abe Y, Yoshida K, Ishii S. A Novel, Potent, and Long-Lasting Dipeptidyl Peptidase-4 Inhibitor, Teneligliptin, Improves Postprandial Hyperglycemia and Dyslipidemia After Single and Repeated Administrations. Eur J Pharmacol (2012) 696(1-3):194–202. 10.1016/j.ejphar.2012.09.024 [DOI] [PubMed] [Google Scholar]
- 144.Werida R, Kabel M, Omran G, Shokry A, Mostafa T. Comparative Clinical Study Evaluating the Effect of Adding Vildagliptin Versus Glimepiride to Ongoing Metformin Therapy on Diabetic Patients With Symptomatic Coronary Artery Disease. Diabetes Res Clin Pract (2020) 170:108473. 10.1016/j.diabres.2020.108473 [DOI] [PubMed] [Google Scholar]
- 145.Matikainen N, Manttari S, Schweizer A, Ulvestad A, Mills D, Dunning BE, et al. Vildagliptin Therapy Reduces Postprandial Intestinal Triglyceride-Rich Lipoprotein Particles in Patients With Type 2 Diabetes. Diabetologia (2006) 49(9):2049–57. 10.1007/s00125-006-0340-2 [DOI] [PubMed] [Google Scholar]
- 146.Onoue T, Goto M, Wada E, Furukawa M, Okuji T, Okada N, et al. Dipeptidyl Peptidase-4 Inhibitor Anagliptin Reduces Fasting Apolipoprotein B-48 Levels in Patients With Type 2 Diabetes: A Randomized Controlled Trial. PloS One (2020) 15(1):e0228004. 10.1371/journal.pone.0228004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chiba Y, Yamakawa T, Tsuchiya H, Oba M, Suzuki D, Danno H, et al. Effect of Anagliptin on Glycemic and Lipid Profile in Patients With Type 2 Diabetes Mellitus. J Clin Med Res (2018) 10(8):648–56. 10.14740/jocmr3464w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of Sitagliptin Therapy on Postprandial Lipoprotein Levels in Patients With Type 2 Diabetes. Diabetes Obes Metab (2011) 13(4):366–73. 10.1111/j.1463-1326.2011.01362.x [DOI] [PubMed] [Google Scholar]
- 149.Kitao N, Miyoshi H, Furumoto T, Ono K, Nomoto H, Miya A, et al. The Effects of Vildagliptin Compared With Metformin on Vascular Endothelial Function and Metabolic Parameters: A Randomized, Controlled Trial (Sapporo Athero-Incretin Study 3). Cardiovasc Diabetol (2017) 16(1):125. 10.1186/s12933-017-0607-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as Yin and Yang of Intestinal Lipoprotein Production: Evidence for Predominance of GLP-2-Stimulated Postprandial Lipemia in Normal and Insulin-Resistant States. Diabetes (2013) 62(2):373–81. 10.2337/db12-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, et al. Dipeptidyl-Peptidase-IV Inhibition Augments Postprandial Lipid Mobilization and Oxidation in Type 2 Diabetic Patients. J Clin Endocrinol Metab (2009) 94(3):846–52. 10.1210/jc.2008-1400 [DOI] [PubMed] [Google Scholar]
- 152.Noguchi K, Hirota M, Miyoshi T, Tani Y, Noda Y, Ito H, et al. Single Administration of Vildagliptin Attenuates Postprandial Hypertriglyceridemia and Endothelial Dysfunction in Normoglycemic Individuals. Exp Ther Med (2015) 9(1):84–8. 10.3892/etm.2014.2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ashraghi MR, Pagano G, Polychronis S, Niccolini F, Politis M. Parkinson's Disease, Diabetes and Cognitive Impairment. Recent patents endocrine Metab Immune Drug Discovery (2016) 10(1):11–21. 10.2174/1872214810999160628105549 [DOI] [PubMed] [Google Scholar]
- 154.Chen S, Zhou M, Sun J, Guo A, Fernando RL, Chen Y, et al. DPP-4 Inhibitor Improves Learning and Memory Deficits and AD-Like Neurodegeneration by Modulating the GLP-1 Signaling. Neuropharmacology (2019) 157:107668. 10.1016/j.neuropharm.2019.107668 [DOI] [PubMed] [Google Scholar]
- 155.Nassar NN, Al-Shorbagy MY, Arab HH, Abdallah DM. Saxagliptin: A Novel Antiparkinsonian Approach. Neuropharmacology (2015) 89:308–17. 10.1016/j.neuropharm.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 156.Wicinski M, Gorski K, Walczak M, Wodkiewicz E, Slupski M, Pawlak-Osinska K, et al. Neuroprotective Properties of Linagliptin: Focus on Biochemical Mechanisms in Cerebral Ischemia, Vascular Dysfunction and Certain Neurodegenerative Diseases. Int J Mol Sci (2019) 20(16):4052. 10.3390/ijms20164052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma M, Hasegawa Y, Koibuchi N, Toyama K, Uekawa K, Nakagawa T, et al. DPP-4 Inhibition With Linagliptin Ameliorates Cognitive Impairment and Brain Atrophy Induced by Transient Cerebral Ischemia in Type 2 Diabetic Mice. Cardiovasc Diabetol (2015) 14:54. 10.1186/s12933-015-0218-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hardigan T, Abdul Y, Ergul A. Linagliptin Reduces Effects of ET-1 and TLR2-Mediated Cerebrovascular Hyperreactivity in Diabetes. Life Sci (2016) 159:90–6. 10.1016/j.lfs.2016.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hardigan T, Yasir A, Abdelsaid M, Coucha M, El-Shaffey S, Li W, et al. Linagliptin Treatment Improves Cerebrovascular Function and Remodeling and Restores Reduced Cerebral Perfusion in Type 2 Diabetes. Am J Physiol Regul Integr Comp Physiol (2016) 311(3):R466–77. 10.1152/ajpregu.00057.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Darsalia V, Mansouri S, Ortsater H, Olverling A, Nozadze N, Kappe C, et al. Glucagon-Like Peptide-1 Receptor Activation Reduces Ischaemic Brain Damage Following Stroke in Type 2 Diabetic Rats. Clin Sci (Lond) (2012) 122(10):473–83. 10.1042/CS20110374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chaturvedi M, Kaczmarek L. Mmp-9 Inhibition: A Therapeutic Strategy in Ischemic Stroke. Mol Neurobiol (2014) 49(1):563–73. 10.1007/s12035-013-8538-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lozhkin A, Vendrov AE, Pan H, Wickline SA, Madamanchi NR, Runge MS. NADPH Oxidase 4 Regulates Vascular Inflammation in Aging and Atherosclerosis. J Mol Cell Cardiol (2017) 102:10–21. 10.1016/j.yjmcc.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rosengren A, Hawken S, Ôunpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of Psychosocial Risk Factors With Risk of Acute Myocardial Infarction in 11 119 Cases and 13 648 Controls From 52 Countries (the INTERHEART Study): Case-Control Study. Lancet (2004) 364(9438):953–62. 10.1016/s0140-6736(04)17019-0 [DOI] [PubMed] [Google Scholar]
- 164.Steptoe A, Kivimaki M. Stress and Cardiovascular Disease. Nat Rev Cardiol (2012) 9(6):360–70. 10.1038/nrcardio.2012.45 [DOI] [PubMed] [Google Scholar]
- 165.Du X, Dong J, Ma C. Is Atrial Fibrillation a Preventable Disease? J Am Coll Cardiol (2017) 69(15):1968–82. 10.1016/j.jacc.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 166.Lei Y, Hu L, Yang G, Piao L, Jin M, Cheng X. Dipeptidyl Peptidase-IV Inhibition for the Treatment of Cardiovascular Disease- Recent Insights Focusing on Angiogenesis and Neovascularization. Circ J (2017) 81(6):770–6. 10.1253/circj.CJ-16-1326 [DOI] [PubMed] [Google Scholar]
- 167.Lei Y, Yang G, Hu L, Piao L, Inoue A, Jiang H, et al. Increased Dipeptidyl Peptidase-4 Accelerates Diet-Related Vascular Aging and Atherosclerosis in ApoE-Deficient Mice Under Chronic Stress. Int J Cardiol (2017) 243:413–20. 10.1016/j.ijcard.2017.05.062 [DOI] [PubMed] [Google Scholar]