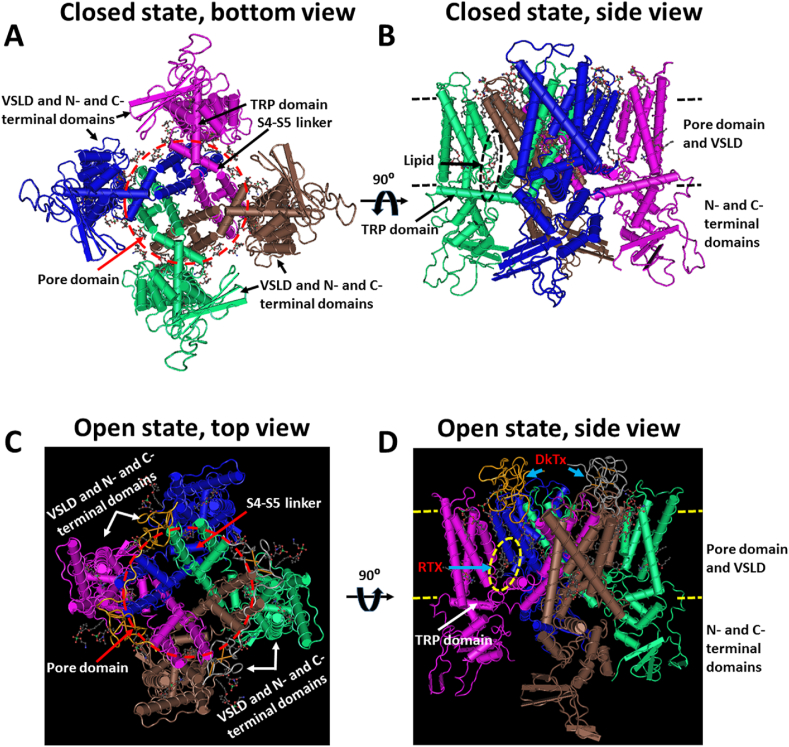
Fig. 1.
The overall cryo-EM structure of TRPV1. The bottom (A) and side (B) views of the homotetrameric arrangements of channel subunits are shown in the cryo-electron microscopy (EM) structures of TRPV1 with four resident phosphatidylinositol lipids bound for the closed state (PDB ID: 5IRZ) [3]. In contrast, the top (C) and side (D) views of the homotetrameric arrangements of channel subunits are indicated in the cryo-EM structures of TRPV1 with two spider toxin DkTx and four resiniferatoxin (RTX) bound for the open state (PDB ID: 5IRX) [4]. Four subunits with different colors form a tetramer. The positions of the lipid bilayer are shown with black (B) and yellow (D) dotted lines. The extracellular side starts from the top line while the cytoplasmic side ends in the bottom line. Four transmembrane helices S1–S4 serve as a voltage sensor-like domain (VSLD) while other two transmembrane helices S5–S6 function as a pore domain (red circled). The pore domain is circled by the VSLD in a domain-swapped arrangement via the S4–S5 linker (A, C). The cation conductance pathway centers in the pore domain and an intervening pore loop region (A, C). A wide outer vestibule with a short flexible selectivity filter is cradled by the VSLD domain. Channel opening is controlled by downward movement of the pore helix between S5 and S6 and coupled dilation of both a selectivity filter and a hydrophobic constriction at the lower S6 gate. The conserved TRP domain links not only the S6 bundle crossing gate but also interacts with the S4–S5 linker promoting allosteric coupling between channel domains. The characteristic ankyrin repeats within the cytoplasmic N terminus facilitate channel assembly by tethering cytoplasmic N- and C-terminal domains [3,4]. A yellow circle is one of four RTX binding sites (D), which share the same location with four resident lipid binding sites, one of which is indicated by a black circle (B). The RTX or lipid binding pocket is formed by the S3 and S4 transmembrane segments, the S5 and S6 segments from a neighboring subunit, and the S4–S5 linker. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)