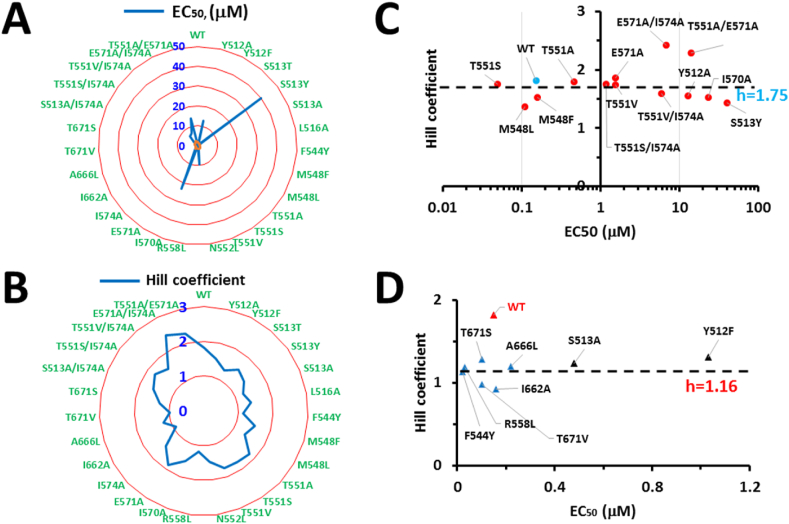
Fig. 4.
The residue site-dependent Hill coefficients and potency of a capsaicin dose response for mTRPV1. A, Radar scanning for the site mutation-induced changes in the capsaicin potency (EC50) of mTRPV1. B, Radar scanning for the site mutation-induced changes in the Hill coefficient of a capsaicin dose response of mTRPV1. Upon radar scanning, the residues in the lipid pocket can be divided into two populations as shown in panels C and D on the basis of the effects of their site mutations on the changes in the Hill coefficient and the capsaicin potency. C, The residues for the non-swapping vanilloid bridge via the side chains between T551 on S4 and E571 on the S4–S5 linker are not cooperative. Their site-directed mutations dramatically change the capsaicin potency (EC50) of mTRPV1 but do not significantly alter the Hill coefficient of a capsaicin dose response. WT is a control colored in blue and other mutants are colored in red. D, The lipid-free residues for the swapping vanilloid bridge via the side chains between T671 on S6 and F544 on S4 are cooperative. Their site-directed mutations significantly decrease the Hill coefficient of a capsaicin dose response but do not dramatically alter the capsaicin potency (EC50) of mTRPV1. WT is a control colored in red and other mutants are colored in blue. Data were produced from the published article [5]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)