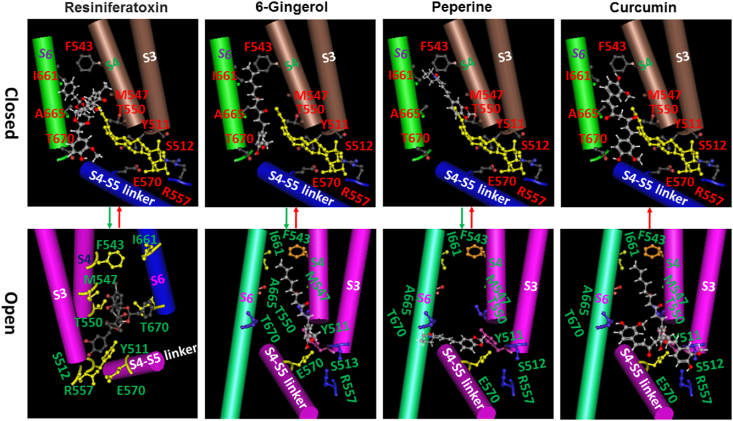
Fig. 6.
The tentative lipid-dependent and anchor-stereoselective interactions between mTRPV1 and vanilloid compounds favor channel opening in a sequential cooperative mechanism. The in silico protomer models were based on the cryo-EM structures of the rTRPV1 channel with a resident phosphatidylinositol lipid bound (PDB ID: 5IRZ) for the closed state or with capsaicin (PDB ID: 3J5R) or RTX (PDB ID: 5IRX) bound for the open state [3,4]. For convenience, only one vanilloid pocket is shown. S3, S4 and the S4–S5 linker are from one subunit while S6 is from the other neighboring subunit. The different rotamers of the residues in the putative binding pocket of TRPV1 were tested to optimally interact with the introduced agonists or antagonists in Fig. 2. The different vanilloid bridges between two separated active residues in Fig. 3 were also examined by the test molecules to make sure their spatial hindrance in the pocket to be minimal but their non-covalent interactions with nearby residues in the pocket to be maximal. In the primary closed state, the inositol ring of a phosphatidylinositol (PI) lipid (yellow) is anchored against S3 and the elbow of the S4–S5 linker, and further stabilized by polar interactions of the side chain of R557 on S4 with the OH- group of the phosphate on position 1, or of the side chain of E570 in the S4–S5 linker with a OH- group on position 6 of the inositol ring, and other electrostatic interactions (not shown). Lipid-free T670 on S6 may anchor the vanillyl head of RTX or 6-Gingerol/capsaicin while lipid-free T550 on S4 may act as an anchor for the vanillyl head of peperine to bind against. In this way, their long tail can be sandwiched via the inter-subunit side chains between F543 on S4 and I661 on S6 so that a recessive silent transient swapping vanilloid bridge can be formed to compete off the resident lipid. In contrast, curcumin has another large vanillyl group and thus cannot form the same bridge as 6-Gingerol does to compete off the resident lipid. When the resident lipid is released, a dominant steady-state stimulatory vanilloid bridge may be established via the intra-subunit side chains between the anchor T550 on S4 and S512 on S3 for RTX or E570 on the S4–S5 linker for 6-Gingerol/capsaicin. In that way, the reorientated Y511 ring may form a face-to-edge π−π interaction for the vanillyl ring to close the binding pocket so that the S4–S5 linker may be uplifted away from the S6 gate for channel opening. In contrast, the swapping peperine bridge between the hydroxyl group of Y511 on the S2–S3 linker and the anchor T670 on S6 may be exploited to uplift the S4–S5 linker away from the S6 gate for channel opening. In the open state, a silent swapping curcumin bridge via the side chains between the anchor T670 on S6 and S512 on S3 or R557 on S4 may compete off capsaicin or 6-Gingerol to inhibit TRPV1 opening. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)