Abstract
Radiotherapy plays an important role in the multidisciplinary management of breast cancer. Recent years have seen improvements in breast cancer survival as well as a greater appreciation of potential long-term morbidity associated with the dose and volume of irradiated organs. Proton therapy reduces the dose to non-target structures while optimizing target coverage. However, there remain additional financial costs associated with proton therapy, despite reductions over time, and studies have yet to demonstrate that protons improve upon treatment outcomes achieved with photon radiotherapy. There remains considerable heterogeneity in proton patient selection and techniques, and the rapid technological advances in the field have potential to impact evidence evaluation given the long latency period for breast cancer radiotherapy recurrence and late effects. In this consensus statement we assess the data available to the radiation oncology community of proton therapy for breast cancer, provide expert consensus recommendations on indications and technique, and highlight ongoing trials cost-effectiveness analyses, and key areas for future research.
INTRODUCTION
Proton therapy (PT) for breast cancer has seen rapid growth due to improved access with expansion of proton centers across the globe, technical advances, and an increasing recognition of the potential late sequelae of breast radiotherapy in survivors. Proton beams have unique physical properties that enable reductions in dose deposition outside of the clinical target volume (CTV). Although patient-specific factors such as comorbidities and genetic makeup modify risk, the dose and volume of radiotherapy to individual organs are important determinants of normal tissue complication probability1. In addition, PT can improve target coverage because many breast cancer photon plans do not deliver the full prescribed dose to the CTV, especially the internal mammary chain, in order to limit heart and lung exposure2–4. Although the dosimetric advantages of PT are well-established, long-term clinical outcomes are only recently emerging. Conducting adequately powered randomized clinical trials directly comparing proton and photon therapy presents unique logistical, financial and medical-ethical challenges related to factors such as the rapid advances in proton and photon therapy technology, evidence of a dose-response relationship for cardiopulmonary toxicity from breast cancer radiotherapy, and prolonged latency of late effects5–10. The full potential and value of PT for breast cancer has yet to be realized. This evidence-based review from the breast subcommittee of the Particle Therapy Co-operative Group (PTCOG) summarizes the potential applications, published clinical data, optimal techniques and ongoing research initiatives of PT in the management of breast cancer.
METHODS
The Institute of Medicine’s Standard for Developing Trustworthy Clinical Practice Guidelines, which recommend that guideline development is led by a multidisciplinary expert panel with transparency where both conflicts of interest and funding sources are reported, were used as a model11. The panel was made up of a diverse international team of physicists and radiation oncologists with expertise in multimodality treatment of breast cancer, including proton therapy. In addition, the panel included one breast cancer survivor treated with photon radiotherapy and a second breast cancer survivor treated with PT, both well informed on both modalities prior to their treatment. All financial relationships and potential conflicts of interest were individually reported. There was no financial support for the development of the guidelines, outside of acknowledgement of protected research time as part of K12 HD065987 training grant (RWM).
The English language scientific articles were identified within a PubMed, MEDLINE, and EMBASE literature search. There were no date restrictions applied. Additional relevant articles were identified through targeted literature searching, reference lists of identified papers or by direct input from the authors. All articles were screened for suitability by the authors. To be included all articles were required to display sufficient detail on methodology, the patient cohorts, and clinical endpoints. Panel recommendations are based on literature evidence, where possible, and clinical experience, where appropriate11. Recommendations were refined in order to obtain the highest possible agreement among the experts. The minimum threshold for inclusion was pre-defined as 75% agreement among the authors. The entire panel contributed to the development of the guidelines and recommendations, provided critical review and approved the final manuscript.
EVIDENCE RATING AND LIMITATIONS
The review is intended to provide guidance to practicing physicians and is not a substitute for professional judgement of individual providers. Level of evidence (LE) supporting recommendations and grade of recommendation was scored according to the Oxford Centre for Evidence-based Medicine (OCEBM) Levels of Evidence Working Group “The Oxford Levels of Evidence 2” (2016 version). A summary of the levels of evidence includes the following: Level 1, systematic review or meta-analysis; Level 2, randomized trial or observational study with dramatic effect; Level 3, non-randomized, controlled cohort or follow-up study; Level 4, case series, case-control or historically controlled study; Level 5, mechanism-based reasoning. A summary of grades of recommendation includes the following: A, consistent level 1 studies; B, consistent level 2 or 3 studies or extrapolations from level 1 studies; C, level 4 studies or extrapolations from level 2 or 3 studies; D, level 5 evidence or troublingly inconsistent or inconclusive studies of any level.
The panel acknowledges that there is no level 1 or level 2 evidence to support proton therapy for pediatric or adult malignancies, including breast cancer. Interpretation of the data must be met with caution, and all recommendations that follow are based on level 3, 4, and 5 evidence. In addition, the pace of technological advances, such as the transition from aperture and compensator-based delivery to pencil-beam scanning techniques, and increasing clinical experience is leading to rapid changes in PT planning and delivery. Thus, clinical trial outcomes may not reflect current practice.
RATIONALE: DOSIMETRIC ADVANTAGES TO ORGANS AT RISK
Cardiac toxicity
Recommendation:
There is a linear relationship between cardiac dose and major coronary events (MCEs) and no threshold below which there is an absence of risk. Therefore, cardiac sparing techniques should be employed for breast radiotherapy. PT reduces the dose to the heart compared to conventional radiotherapy (LE 3, Grade B).
Mean heart dose has been a principal planning parameter used in breast cancer radiotherapy since a seminal study reported a linear relationship between mean heart dose and MCEs12. For each 1 Gy increase in mean heart dose, a 7.4% relative increase in MCEs was observed. This translated to an estimated 0.3–0.6% per Gy absolute increase in MCEs by age 80, with variation mediated by the presence of cardiac risk factors and age at radiotherapy. No threshold was identified below which there was an absence of risk12. These findings have been validated by others13. The dose to the left ventricle and coronary artery subsegments have also been correlated with major coronary events13–15, and correlations between cardiac dose, right ventricular systolic dysfunction and development of heart failure with preserved ejection fraction have been reported16,17. Cardiac sparing is of particular importance in patients with baseline cardiac risk factors. Indeed, increasing age, presence of hypertension, diabetes, hypercholesterolemia and smoking history all increase the absolute risk associated with a given radiation dose to the heart 12,13. Although advances have been made in photon cardiac sparing with techniques such as deep-inspiratory breath hold and intensity modulated radiotherapy (IMRT), the use of cardio-toxic systemic agents including anthracyclines and human epidermal growth factor receptor 2 (HER2) directed therapies may potentiate risk of cardiac exposure20.
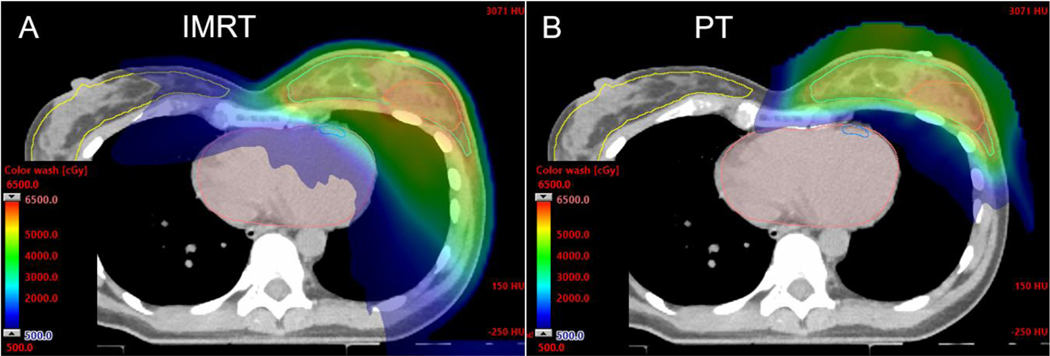
Additional work is needed to better delineate the relationship between cardiac subsegment dose volume parameters, systemic therapy, and host factors with various cardiac endpoints21. In the future, the application of early markers of radiation-associated cardiac damage may enable improved prediction and ultimate mitigation of late cardiac toxicity from breast radiotherapy22,23. The available literature supports strategies to limit dose to all segments of the heart in order to reduce late radiation-related cardiac morbidity14. PT reduces the dose to the heart compared with 3-dimensional conformal radiotherapy (3DCRT) and intensity modulated radiotherapy (IMRT, Table 1, Figure 1) 5,20,24–32.
Table 1.
Dosimetric analyses of proton therapy for regional nodal irradiation (RNI).
| Author | Technique | Laterality | Target | Heart | Lung | Contralateral Breast | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Ares (27) | 3D 1M IP |
Left (n=20) WB (n=10) CW (n=10) | Metric (% Rx Dose) | 3D | IM | IP | Metric (% Rx Dose) | 3D | IM | IP | Metric (% Rx Dose) | 3D | IM | IP | Metric (%Rx Dose) | 3D | IM | IP |
| Max | 121* | 112 | 109 | Mean | 19 | 19 | 4 | L Mean | 41 | 26 | 14 | Max | 15–84 | 42 | 12 | |||
| Mean | 102 | 101 | 101 | V22.5 | 11 | 8 | 8 | LV20 | 39 | 21 | 15 | Mean | 3 | 10 | 0 | |||
| Min | 94 | 93 | 98 | V5 | 40 | 68 | 12 | LV5 | 76 | 80 | 28 | |||||||
| D95 | 94 | 93 | 98 | R Mean | 3(CW) 55 (WB) | 13 | 1 | |||||||||||
| V95 | 95 | 2 | 100 | R V20 | 0 | 1 | 1 | |||||||||||
| VI07 | 17 | 2 | 1 | RV5 | 1 | 63 | 1 | |||||||||||
| Hernandez | VM | Left (n=8) | Metric (%Rx | VM | PP | IP | Metric (%Rx | VM | PP | IP | Metric | VM | PP | IP | Metric | VM | PP | IP |
| (28) | PP IP |
Dose) | Dose) | (%Rx Dose) | (%Rx Dose) | |||||||||||||
| D95 (Gy) | 47.8 | 48.6# | 49.4 | V5 | 88 | 2.02 | 3.05 | V5 | 53.2 | 13.1 | 19.0 | V5 | 57 | 0.99 2.1 | ||||
| V107 | 0.2 | 0.1 | 0.003 | V22.5 | 12 | 0.49 | 0.56 | V20 | 14.7 | 8.73 | 8.72 | Mean | 7.3 | 0.17 0.34 | ||||
| DHI | 0.13 | 0.09 | 0.58 | |||||||||||||||
| Cl | 0.72 | 0.81 | 0.86 | |||||||||||||||
| Jimenez | P/E | Left CW | Metric (%Rx | P/E | WT | IP | Metric (%Rx | P/E | WT | IP | Metric ( c/( Rx | P/E | WT | IP | ||||
| (31) | WT | (n=5) | Dose) | Dose) | Dose) | |||||||||||||
| IP | Right CW | Mean to PTV | 99 | 100 | 100 | LV5 | 26.6 | 19.7 | 2.8 | LV5 | 53.6 | 49.2 | 14.9 | |||||
| (n=5) | Max | 133 | 120 | 115 | L V10 | 15.8 | 16.2 | 1.0 | L V10 | 43.6 | 42.2 | 9.5 | ||||||
| D2 | 124 | 116 | 114 | L V20 R V5 R V10 RV20 | 7.8 11.5 4.6 1.2 |
13.6 1.2 0.5 0.3 |
0.4 0.3 0.1 0.1 |
LV20 RV5 RV10 RV20 |
31.9 65.1 52 36.4 |
36.7 44.6 38.3 |
4.3 13.5 8.6 4.1 |
|||||||
| MacDonald | P/E | Left CW (n=l 1 ) | Metric (%Rx | P/E | WT | PP | Metric | P/E | WT | |||||||||
| (32) | WT PP |
Dose) | (%Rx Dose) |
|||||||||||||||
| PP | Metric (%Rx | P/E | WT | PP | ||||||||||||||
| Dose) | ||||||||||||||||||
| V90 92 | 94 | 99 | V5 | 35.6 | 20.9 | |||||||||||||
| 4.1 | L V5 | 46.3 | 33.2 | 25.2 | V95 | 89 | 91 | 98 | ||||||||||
| VI0 22.7 | 14.9 | 2.8 | L VI0 | 32.2 28.5 | 21.3 | |||||||||||||
| V20 | 12.4 | 12 | 1.6 | LV20 | 21.7 | 25.3 | 16.2 | |||||||||||
| V45 2.2 | 6.9 | 0.3 | ||||||||||||||||
| Ranger | WT-DIBH | Left | Metric (%Rx | WT | VM | IP | Metric (Gy) | WT | VM | IP | Metric ( Gy) | WT | VM | IP | Metric | WT | VM | IP |
| (24) | VMAT-DIBH | WB | I)ose) | (Gy) | ||||||||||||||
| IMPT-FB | (n=10) CW (n=4) |
PTV | 85 | 96 | 100 | Mean V17Gy (%) L AD Max |
2.5 2 38.3 |
2.6 1.8 36.2 |
1.0 1.3 17.4 |
V17Gy (%) Mean | 28 0.8 |
28 0.7 |
17 0.2 |
Mean | 1.4 | 1.5 | 0.2 | |
| Flejmer | 3D-DIBH | Left WB | Metric | 3D | UP | IP | Metric | 3D | UP | IP | Metric | 3D | UP | IP | ||||
| (29) | UP-DIBH | or CW | PTV Mean (Gy) | 50.1 | 50.« | 50.0 | Mean (Gy) | 2.1 | 0.2 | 0.2 | L Mean (Gy) | 12.3 | 7.3 | 6.9 | ||||
| IP-DIBH | (n=10) | PTV V95 (%) | 93.9 | 97.1 | 99.0 | D2% (Gy) | 16.7 | 3.2 | 3.2 | LD2% | 46.9 | 32.9 | 33.6 | |||||
| PTV V105 (%) | 5.0 | 0.1 | 0.1 | V20 ( % ) | 1.6 | 0 | 0.1 | L V20(%) | 23.3 | 13.1 | 11.3 | |||||||
| PTV D98 ( % ) | 46.7 | 47.0 | 48.2 | V5 (%) | 4.9 | 1.3 | 1.3 | L V10(%) | 30.1 | 28.8 | 26.8 | |||||||
| PTV 1)2% (Gy) | 52.8 | 51.4 | 51.2 | LAD Mean (Gy) |
14.6 | 3.2 | 3.3 | Integral Dose (Gy) | 114.1 | 67.0 | 67.0 | |||||||
| HI | 12.3 | 8.8 | 6.0 | LAD D2% (Gy) |
29.9 | 10.2 | 10.3 | |||||||||||
| Fagundes | 3D | Left CW | Metric | 3D | VM | IP | Metric | 3D | VM | IP | Metric | 3D | VM | IP | Metric 3D VM | IP | ||
| (30) | VM | (n=10) | PTV V100(%) | 69.2 | 85.8 | 81.9 | Mean (Gy) | 6.8 | 8.2 | 1.2 | L V20 (%) | 40.4 | 27.3 | 28.4 | V5 (%) 0.5 19.7 | 3.8 | ||
| IP | PTV V95 (%) | 86.5 | 95.6 | 91.6 | V25 (%)’ | 6.3 | 7.4 | 1.2 | L V10 (%) | 53.3 | 40.6 | 35.9 | Mean 0.5 3.8 (Gy) | 0.6 | ||||
| PTV V90(%) | 92.2 | 98.4 | 95.8 | LAD Mean (Gy) |
20.9 | 15.5 | 7.0 | L V5 (%) | 66.4 | 58 | 41.3 | |||||||
Abbreviations: VM = volumctric nnxiulatcd arc therapy: PP = passively scattered proton therapy: WT = 3D conformai wide tangents: IM = intensity modulated radiation therapy: IP = intensity modulated proton therapy: UP = single field uniform proton therapy: WB = whole breast; CW = chest wall, 3D-CRT = 3D conformai radiotherapy: DH1 = dose homogeneity index: Cl = conformity index; DIBH = deep inspiratory breast hold: Gy = Gray (Relative Biological Effectiveness [RBE]); LAD = left anterior descending artery.
When a range was given, the average value is shown.
Bolded and italicized values have an associated p-value less than 0.05, indicating a statistically significant difference versus the comparative group, when these data were available.
Figure 1:
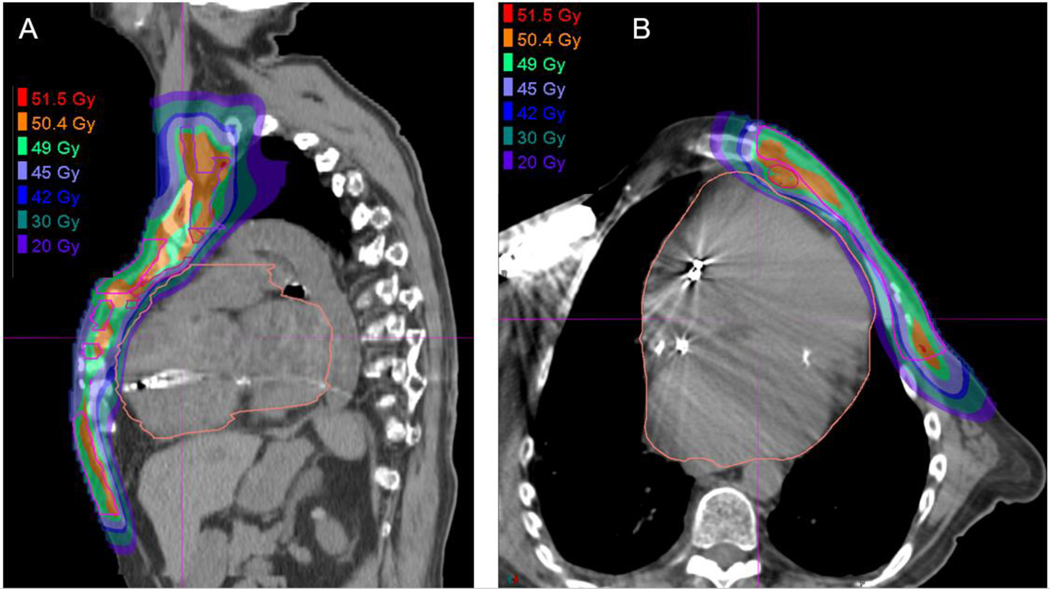
Axial CT dose color wash from an IMRT plan (A) and multi-field optimized PBS PT plan (B) for whole breast and RNI demonstrating improved heart, lung, contralateral breast, and other soft tissue sparing with PT. The lumpectomy cavity CTV is highlighted in orange where a simultaneous integrated boost is administered to 56.26 Gy in 25 fractions, with the breast and nodal volumes (not shown) receiving 50 Gy.
Radiation pneumonitis
A dose-volume relationship for radiation pneumonitis is established, and similar to radiation-induced coronary artery disease, there is no well-defined threshold under which the risk of toxicity disappears33. In a breast cancer systematic review and meta-analysis, predictors of radiation pneumonitis included supraclavicular fossa irradiation, older age (age > 55 years), ipsilateral lung V20Gy >30% and ipsilateral mean lung dose > 15 Gy 34. Patients with comorbidities such as interstitial lung disease that increase the risk of radiotherapy-related pulmonary complications or simultaneously receiving systemic agents associated with pneumonitis may especially benefit from pulmonary sparing35,36. PT reduces the dose to the lungs in early stage and locally advanced breast cancer compared with 3DCRT and IMRT 24,25,33,37–39.
Clinical pneumonitis is a rare complication of breast cancer radiotherapy with PT and photon therapy40,41. In a prospective PT trial of 69 patients treated to the breast or chest wall and regional lymph nodes with PT, the primary endpoint was incidence of grade ≥3 radiation pneumonitis or any grade 4 toxicity within 3 months of radiotherapy41. There were no grade ≥ grade 3 pneumonitis events. One patient developed grade 2 radiation pneumonitis and was treated successfully with oral steroids41. Enhanced linear energy transfer (LET) at the end of the proton range, as described in the section below, could lead to enhanced proton relative biologic effectiveness (RBE) for pneumonitis and should be taken into consideration during treatment planning42–44. Though to date, clinical data does not suggest a difference, large prospective studies will be needed to reliably compare pneumonitis rates across modalities.
Lung cancer
The risk of secondary lung cancer in patients undergoing breast cancer radiotherapy is also associated with dose to the lungs5,45. Taylor et al. reported an excess rate ratio for incident lung cancer of 0.11 per Gy mean lung dose5. The absolute risk was particularly pronounced in active smokers5. Grantzau et al. reported that second primary lung cancer five years or more after breast cancer radiotherapy increased linearly by 8.5% per Gray; the increase in lung cancer risk was 17.5% per Gray for those with smoking histories45. Thus, strategies that reduce lung exposure are especially important in current and former smokers. Both low and high dose components of the dose volume histogram for the lungs are reduced with PT25, and dose modeling studies have suggested a lower risk of second lung cancer following PT compared with modern photon radiotherapy techniques5,46.
Arm and shoulder function
Arm and shoulder motion impairment can result from breast and axillary surgery and/or radiotherapy, and the combination of surgery and radiotherapy appear to have the greatest functional impact47–50. The dose to the humeral head, scapula, and muscles involved in arm and shoulder movement are all reduced with PT51. Carefully designed prospective quality of life studies are warranted to test the hypothesis that PT can improve arm and shoulder functional outcomes in patients undergoing axillary and supraclavicular irradiation.
Contralateral breast cancer
Recommendation: The mean dose to all quadrants of the contralateral breast should be limited to < 1 Gy, particularly in women < 40 years (LE 4, Grade C).
Dose to the contralateral breast increases the risk of developing second primary contralateral breast cancer, with risk being inversely related to age at exposure 46,52,53. For example, in the Women’s Environmental, Cancer, and Radiation Epidemiology (WECARE) study, women < 40 with quadrants of breast exposed to > 1.0 Gy mean dose had a 2.5-fold greater risk of contralateral breast cancer than unexposed women52.
Minimizing contralateral breast exposure is an important objective of breast radiotherapy planning, but may be especially challenging when administering internal mammary node (IMN) irradiation, treatment to medially located primary tumors, and in women with large contralateral breasts (Figure 1). Beyond dose and young age, breast cancer family history and pathogenic germline alterations in breast cancer susceptibility genes such as BRCA1, BRCA2, PALB2, CHEK2, p53 and ATM have also been associated with an increased lifetime risk of contralateral second primary breast cancer, as well as other secondary malignancies 52–56. The beam arrangements standardly used for proton therapy allows for near complete sparing of the contralateral breast to any dose of radiation. By reducing exposure to the contralateral breast, modelling studies suggest that PT will reduce the lifetime risk of contralateral second primary breast cancer over 3DCRT and IMRT 46,57,58.
Other secondary malignancy
Recommendation: Patients with germline mutations that increase susceptibility to radiation-induced second cancers and are indicated to receive adjuvant radiotherapy should be considered for PT (LE 4, Grade C).
Pooled data from individual patients treated in historical trials suggest that breast cancer radiotherapy, as administered using the technology available at that time, was associated with increased risk of soft tissue sarcoma and esophageal cancer59. PT reduces soft tissue exposure outside of the CTV, and a modelling study has suggested reduced risk of soft tissue sarcoma following PT for breast cancer compared with 3DCRT and IMRT46. In addition, an analysis of patients in the National Cancer Database with a first cancer diagnosis of 9 tumor types between 2004–2015 who received 3DCRT, IMRT, or PT was reported. Among patients with breast cancer who had > 5 years follow-up, PT was associated with a significantly lower risk of second cancer compared with IMRT (adjusted odds ratio 0.62, 95% confidence interval, 0.41–0.95; p=0.029)60. Germline mutations in p53, RB1, and NF1, which result in Li-Fraumeni syndrome, hereditary retinoblastoma, and neurofibromatosis type I, respectively, increase the risk of radiation-induced second cancers56. The benefits of normal tissue sparing may be particularly pronounced in these patients when therapeutic radiation is indicated61.
Importantly, the dose to the esophagus can be increased with volumetrically planned radiotherapy as a result of more generous target coverage26,46. Therefore, careful supraclavicular CTV delineation as described below, and application of strict esophageal constraints are critical to limit the risk of esophageal toxicity and secondary malignancy with modern radiotherapy, including PT26,46,62.
TREATMENT PLANNING CONSIDERATIONS, UNCERTAINTIES AND MITIGATION
Targeting
Recommendation: Meticulous, evidence-based CTV delineation is of high importance in PT treatment planning due to steep dose gradients (LE 5, Grade D).
Precise CTV delineation all-inclusive of sites at risk of harboring microscopic disease is of high importance in PT due to the sharp dose fall off at the PT Bragg peak6,63. For example, although the RTOG breast cancer atlas does not include coverage of the posterolateral supraclavicular fossa (i.e. the posterior triangle), historically delivered conventional anterior-oblique supraclavicular photon therapy fields deliver a considerable exit dose into this region. This exit dose into the posterior triangle is often at a magnitude that is expected to control microscopic disease due to the slow attenuation of photons through tissue64. Studies suggest that the posterior triangle is a common site of supraclavicular nodal metastases, with approximately half of supraclavicular nodes located marginal to or outside of the RTOG CTV in the posterior and lateral direction65–68. Therefore, more generous target delineation in this area should be considered in patients with indications for regional nodal irradiation (RNI), such as defined in the atlas for the RADCOMP trial (RTOG 3510)69. In contrast, paraesophageal and paratracheal nodes are infrequent sites of breast cancer nodal metastases65–68. In the absence of clinical nodal involvement, the medial border of the CTV should not extend medial of the internal jugular vein70, and an esophageal constraint should be applied during plan optimization in order to limit the risk of esophagitis and secondary esophageal cancer 37,70,26,46,59. In cases for which gross adenopathy was visible on initial staging work-up, fusion of the pre-treatment PET and/or CT image sets with the CT simulation images can ensure that the high risk nodal areas are encompassed in the CTV. For the chest wall CTV, the posterior border should not extend into the intercostal muscles or ribs in the absence of direct clinical invasion, which enables rapid PT dose fall off anterior to the heart and lungs and reduced dose to the ribs (Figure 2A-C)71.
Figure 2:
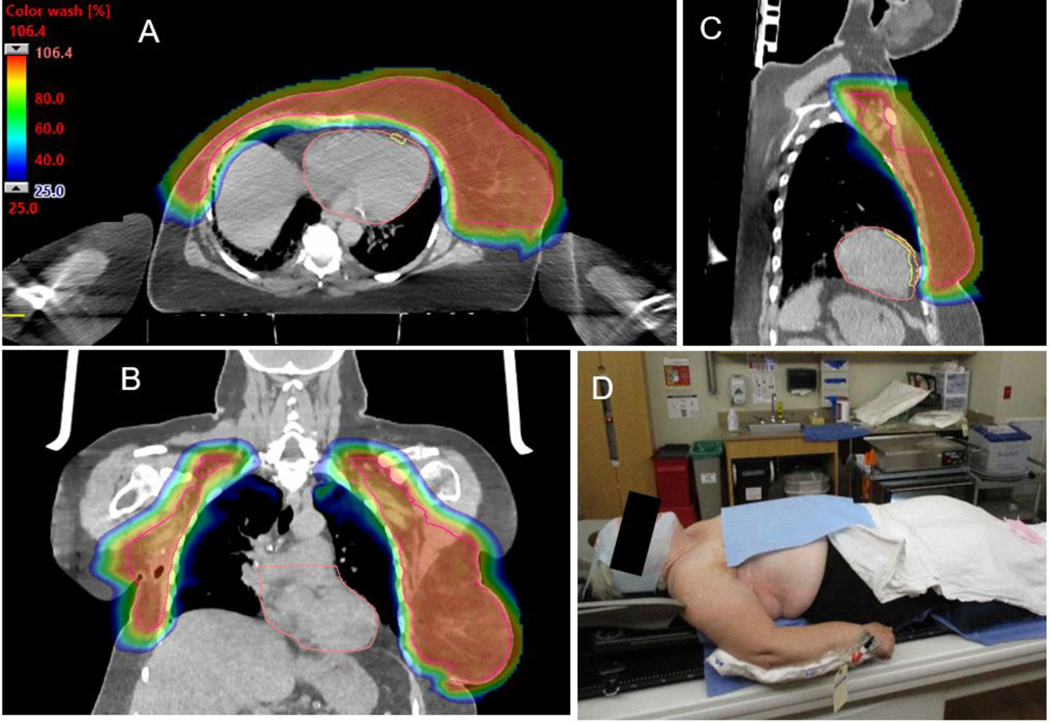
Axial (A), coronal (B), and sagittal (C) 25% color wash images demonstrating comprehensive CTV coverage (pink) with a homogeneous dose (maximum dose 106.4% of prescription) and excellent normal tissue sparing for recurrent breast right breast cancer with ipsilateral and contralateral axillary metastases. (D) Medical comorbidities necessitated an arms down immobilization. The arm is in an akimbo position to attempt to maximize separation between the arm and CTV.
With the evolution of PMRT contouring guidelines, PT may be poised to further limit normal tissue exposure compared with photon delivery techniques. For example, ESTRO has recently revised its consensus guidelines for PMRT target delineation in the setting of immediate breast reconstruction. In this guideline, the chest wall CTV for implants in the retropectoral location is limited to the subcutaneous tissue ventral to the implant and pectoralis major muscle, and excludes the tissues posterior to the implant in the absence of “adverse factors” in patients undergoing PMRT72. If the safety of such an approach is ultimately confirmed prospectively, PT would be ideally suited to deliver radiation to these smaller volumes, thereby further reducing exposure to the heart, lungs, and ribs. However, recurrences within and posterior to the pectoralis muscles have been reported, and may be clinically occult71,73. Further, this area has been routinely treated in PMRT trials, and is at least partially covered in photon plans, even if not specifically delineated in the CTV. Finally, this technique has not been tested in the setting of pre-pectoral implant reconstruction, an increasingly common reconstruction technique37,74,75. Therefore, inclusion of the most posterior extent of the implant and immediately adjacent tissue anterior to the intercostal muscles and ribs in the PT chest wall CTV may be considered based on the currently available data71,76.
LET and RBE heterogeneity
Recommendation: Preclinical and clinical evidence suggests that the proton RBE for tumor control and toxicity is increased at the end of the proton range. Treating physicians should be mindful of these potential effects during treatment planning (LE: 4, Grade C).
In clinical practice, a fixed proton RBE (the dose of photons divided by the physical dose of protons to have the same biologic effect) of 1.1 is routinely employed. However, the RBE may be greater than 1.1 at the Bragg peak and distal falloff where the LET of the proton beam profile is greatest77. In typical breast or chest wall with RNI planning, the distal edge of the proton beam is located at the ribs and intercostal space, just distal to the target regions but proximal to the critical structures of the heart and lungs. This higher LET may lead to a greater RBE for normal tissue effects and tumor control than predicted when a constant physical dose to proton RBE conversion is assumed78. Indeed, the Massachusetts General Hospital (MGH) group has reported clinical data suggesting an RBE >1.1 for late-phase pulmonary radiographic changes as well as rib fractures41,42,44. At the Mayo Clinic, coverage with 90–95% of the physical prescription dose may be accepted in the most posterior few millimeters of the breast, chest wall, and IMN CTVs due to the higher LET and anticipated biologic range extension at those locations. Two to three fields are routinely employed, in order to limit potential biologic hot spots and biologic range extension into the ribs, chest wall, heart and lungs, and a constraint is applied to the brachial plexus with the goal of mitigating the risk of RBE heterogeneity on late brachial plexopathy43. In the future, advances in PT treatment planning and delivery such as LET/RBE optimization, variable RBE modelling, and Spot-scanning proton arc therapy (SPArc), may further mitigate the potential normal tissue effects of LET/RBE heterogeneity79–82.
Range uncertainty
As with all PT planning, the potential effect of range uncertainty, usually quantified as a percentage of the proton’s incident range, must be considered83,84. The required ranges to treat the typical breast CTV are relatively shallow and most of the traversed tissue for breast treatment consists of fatty, muscular or glandular tissues which have lower uncertainties for range calculations85. Therefore, the composite distal margins that are applied to account for range uncertainly in order to generate robust PT plans are often on the order of 2 to 3 mm, which enables exquisite sparing of the underlying heart and lungs.
Adaptive re-planning
Recommendation: Clinical exam, surface imaging and/or volumetric imaging may identify anatomical changes that impact dosimetry enough to warrant re-planning (LE: 4, Grade C).
Reliable positioning of tissue is essential to maintain the consistent path length of the proton beam. The en face orientation of the proton beam in combination with the finite range of protons make the dose distribution along the chest wall interface sensitive to change. For example, contraction of the breast or chest wall such as from resolution of a seroma will decrease the proton path length, resulting in increased dose to the lung and heart86. In contrast, swelling from treatment can create a layer of under-dosing at the deepest portion of the target along the chest wall or IMN CTV87. Surface imaging can identify interfraction anatomical variation and has also been used for intra-fraction tracking 37,88. In addition, periodic volumetric imaging evaluations with overlaying of the treatment plan may be considered to quantify the effects of anatomical changes during the course of PT87.88 Adaptive re-planning may be considered in instances where tissue changes lead to a breakdown in plan robustness89–92. Robust treatment planning with simulations of potential interfraction anatomical variation can be used to minimize the need for replanning37,93. Thus, in addition to patient-specific factors, the need for adaptive re-planning may vary with planning parameters employed by the treating institution and the robustness of the original plan. In one study, 10% of PMRT patients with reconstruction underwent replanning to improve CTV coverage and/or homogeneity37. However, verification scans were acted upon ≤ 1% of the time at that institution for APBI and have since been abandoned in the absence of clinically apparent tissue changes at the time of treatment compared to simulation (RWM, unpublished observations).
Partial breast planning considerations
Reproducible setup has been achieved for APBI with prone positioning and supine positioning in arms up or down position39,86,94–96. Many of the initial planning techniques used for treatment of proton APBI included 3–4 fields using fixed spread out Bragg peak (SOBP), aperture and compensator-based double scattering 94. Depending on the target position within the breast, the process of avoiding skin overlap with these techniques must be accomplished by using larger hinge angles between treatment fields. These more tangential fields can spare skin dose but treat larger volumes of breast tissue to low doses and demonstrate more sensitivity to alignment errors and respiratory motion97. A patient-specific multi-field approach representing compromise between integral dose, skin sparing, and end-of-range uncertainty is desirable39,97.
The implementation of PBS for APBI provides the planner with spot by spot intensity control of the proton field. This added degree of freedom can be used to optimize proximal conformality to provide skin sparing, even in the case of overlapping enface fields. In addition, advanced spot optimization methods (Multi Field Optimization, MFO) can be used to increase overall plan robustness to set-up and range uncertainties and further reduce OAR doses when compared to the aperture and compensator methods described above95.
Breast/chest wall and RNI planning considerations
To avoid excessive dosing of surrounding tissues, en face fields are most commonly utilized, which allow for distal sparing of the heart and lung and a beam path in the direction of respiratory motion. In this case, the distal fall-off of the Bragg peak is placed within the ribs, intercostal spaces and IMNs where the full advantage of the sharp distal falloff of the proton beam is achieved. Regional lymphatics, including the axillary and supraclavicular nodes, are also treated with enface fields. For aperture and compensator-based PT, the size of the treatment area generally requires multiple matched fields and match line changes. Traditional methods of verifying match lines on the skin surface clinically are difficult with typical PT delivery systems98. The control of spot intensity offered by PBS delivery enables PT delivery without the need for collimator matching. PBS field edges are designed to be insensitive to set-up errors with slow gradient dose fall-off, thus eliminating the need for junction changes which can be particularly advantageous for treatment of large targets, as in bilateral breast cancer (Figure 2). In addition, PBS allows photon-like skin sparing throughout the entire treatment field (Figures 3, 4). In plans where the target dose is optimized to deliver a uniform dose to the entire target region (Single Field, Uniform Dose, SFUD), the maximal magnitude of the skin sparing in the breast/chest wall region is dependent on the modulation across the target, with less surface sparing achievable in regions where large modulations are required. This presents a situation where the maximum extent of skin sparing will be non-uniformly distributed across the treatment field. By using more than a single field for treatment and not requiring a uniform dose from each individual field (MFO), the combined field spot patterns can be optimized together to achieve a more optimal solution with more uniform skin sparing37,93. Given these significant differences for breast cancer PT, attention to the techniques employed (i.e. PBS versus aperture and compensator-based PT treatments) is critical in evaluating and interpreting PT treatment outcomes.
Figure 3:
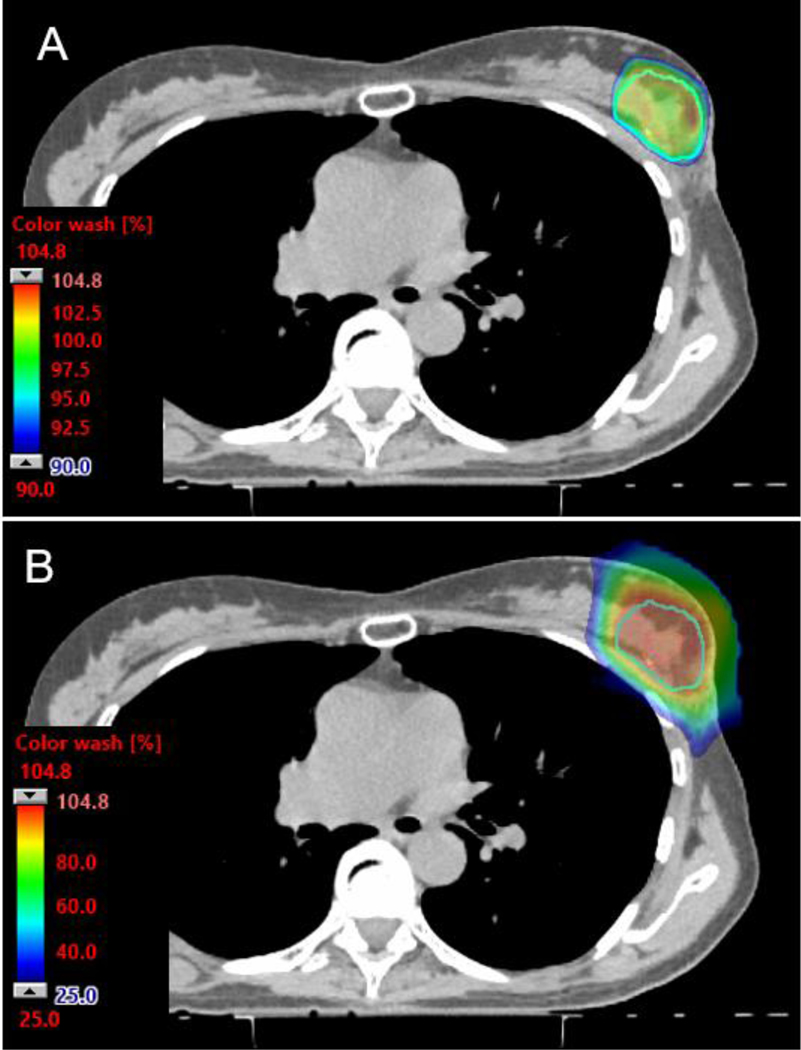
Axial CT dose color wash images from a PBS PT APBI plan. The 90% color wash (A) demonstrates the high level of conformality and skin-sparing properties of the PBS technique. (B) The 25% color wash at the same level demonstrates exquisite normal tissue sparing, including of non-target breast tissue.
Figure 4:
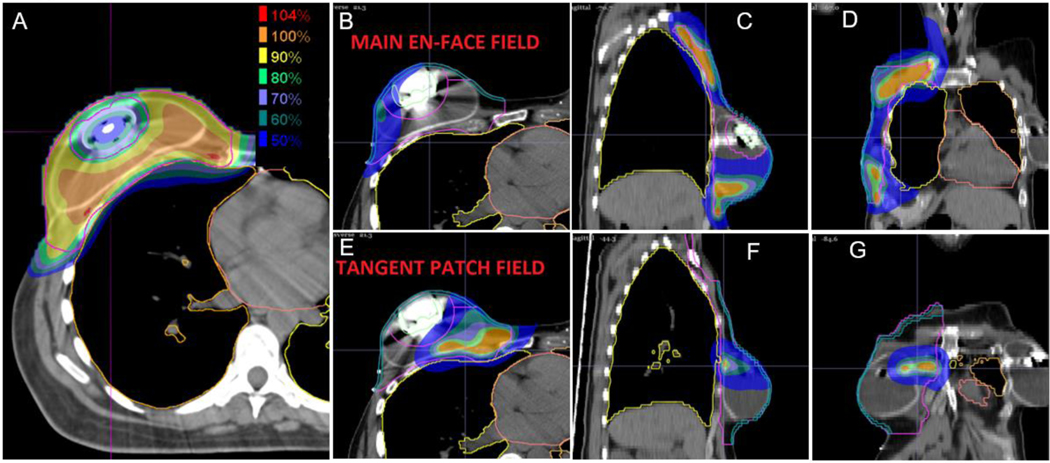
Axial (A, B, E), sagittal (C, F), and coronal (D, G) 50% color wash images demonstrating a two proton field, multi field optimization (MFO) plan avoiding delivery through the magnetic expander port of the reconstructed breast while achieving comprehensive target coverage. Individual dose deposition profiles from the en face field (B-D) and the more tangential beam angle (D-F) are displayed.
Tissue expanders are commonly placed at the time of mastectomy as part of two-stage immediate breast reconstruction. These expanders contain metallic ports made of high atomic number materials which create considerable artifact on the CT planning images, raising the complexity of accurately predicting the stopping powers through and around the port99. Several planning strategies have been implemented to address this challenge including metal artifact reduction algorithms, MFO techniques to treat around the port (Figure 4), and careful characterization of the port and use of Monte Carlo methods to improve accuracy of dose calculation, thus enabling treatment through the port37,99–102. The resulting proton plans have superior dosimetric characteristics over photon plans.99,102
Deep inspiratory breath hold (DIBH) has been shown to be an effective method to reduce heart dose in photon planning by displacing the heart and coronary arteries away from the treatment beams103. DIBH may also facilitate displacement of cardiac structures inferiorly and posteriorly away from the IMN CTV and areas of high LET in some cases of breast PT. However, routine use of DIBH to reduce heart dose in unselected patients does not appear to be beneficial24,104. The physical displacement of the heart created by the DIBH process is usually replaced with low density lung tissues. Since breast proton treatment uses en face proton fields, this results in minimal change of the water equivalent path length to the heart, and typically little additional sparing of the heart is achieved in the absence of significant inferior cardiac displacement. With photon tangents, respiratory motion has the effect of oscillating the deep edges of the target regions in and out of the high dose areas. With the en face fields used in proton delivery, the proton path is typically in the direction of the respiratory movement. Although these tissues are moving as part of the respiratory cycle, the proton path length from the skin to the distal edge of the target does not change significantly with respiratory motion24,105. Thus, typical respiratory patterns are not a major source of intrafraction uncertainty relative to the uncertainties for beam range and setup which should be accounted for during routine treatment planning process105.
CLINICAL EVIDENCE AND INDICATIONS
Regional nodal irradiation
Recommendation: PT is a treatment option for patients with indications for RNI. Patients in whom recognized target coverage and/or organ at risk constraints cannot be achieved with a robust photon plan may be most likely to derive benefit. Clinical trial enrollment should be considered if available (LE: 3, Grade B).
RNI encompassing axillary, supraclavicular, and IMN basins is routinely indicated in lymph node positive, locally advanced, or medially located breast cancers40,63,106–109. Delivery of RNI poses dosimetric challenges due to the large CTV size and location of the CTV in close proximity to the heart, lungs, and other organs at risk. 3DCRT techniques have several well-recognized limitations for RNI. Traditional 4- or 5-field photon and electron 3DCRT approaches introduce uncertainties at match lines and potential for cold spots at abutting fields2. Partially wide tangents and IMRT improve homogeneity but also result in additional compromises, including increased dose to the contralateral breast57,110. IMRT has excellent conformality but increases low and intermediate dose spread to tissues outside of the CTV (Figure 1)46,57,111. Regardless of technique, the dose to organs at risk can only be reduced so much after which compromises in CTV coverage arise, which can lead to suboptimal disease control2–4,40,63,109. PT addresses these limitations. Modern photon techniques such as DIBH and IMRT can typically provide homogeneous target coverage while limiting doses to organs at risk to levels expected to result in low rates of late adverse events. Nevertheless, multiple dosimetric analyses have demonstrated that PT improves CTV coverage, including of the IMNs, while simultaneously decreasing dose to organs at risk and overall integral dose (Table 1).
These promising early PT planning studies spurred a number of prospective clinical studies in patients with indications for RNI (Table 2)25,37,41,112–114. Initial reports from clinical trials from the University of Florida and MGH demonstrated favorable early toxicity outcomes, and confirmed that PT was associated with significantly lower heart and lung dose compared with 3DCRT using either photon-electron matched fields or partially wide tangents25,32,112.
Table 2.
Prospective clinical trials of proton therapy for regional nodal irradiation (RNI)
| Series | Year/ Modality | N/ Stage | Target / Dose | Heart | Lung | Other Metric | Median F/u | Toxidty | Disease Control | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| MGH(4I) | 2011–2016 | 69 | L <n=63) | Metric | PP/PBS | Metric | PP/PBS | Constrain Used | — | Acute: | 5-year | |
| R(n=4) | (Median. Gy) | (Median) | Grade 2 | LRC 983% | ||||||||
| B/l (n=2) | Mean | 03 | Mean (Gy) | 7.7 | Heart | <13 Gy | Dermatitis 83% | OS 91% | ||||
| CW (n=64) | Mean | Dysphagia 7% | ||||||||||
| WB(u=5) | Min | 0.1 | Min (Ĉ) | 0.1 | I.AD Max | <10Gy | Fatigue 35% | |||||
| PP(n=23) | 1(6%) | 45–50.4 Gy | Max | 16j6 | Max (Gy) | 45.9 | I.AD Mean | <2Gy | Grade 3 | |||
| PBS (n=46) | 11(58%) | +/− | I.AD Mean | 1.16 | V20 (%) | 14.5 | Ipsi Ioing V20 | <20% | Dermatitis 4% | |||
| nn (36%) | 14.4 Gy Boost | I.AD Mb | 0.1 | Esophagus Max | <40 Gy | Late: | ||||||
| I.AD Max | 4.7 | Unplannod Sx −33% | ||||||||||
| Grade 1 | ||||||||||||
| Rib fracture 1% | ||||||||||||
| Grade 2 | ||||||||||||
| Pneumonitis 1% | ||||||||||||
| Mayo (37) | 2015–2017 | 51 | CW+Immod. | Metric | IP | Metric | IP | — | 19 mo | Acute: | — | |
| Reconst. | (Median) | (1QR) | (Median) | (IQR) | Grade 2 | |||||||
| L (n=35) | Mean (Gy) | 06 | Ipd Mean (Gy) | 73 | Dermatitis 33% | |||||||
| R (n=14) | (0.4–0.9) | (63–83) | Esophagitis 2% | |||||||||
| B/l (n=2) | Fatigue 2% | |||||||||||
| IP | 1(12%) | 50Gy(n=37) | V25(%) | 0.1 | IpdV20(%) | 13.9 | Dccrcasod Joint ROM 2% | |||||
| 11(49%) | 403Gy(n=14) | (0.0–0.3) | (10.1–14.9) | Br. Plcxop. 2% | ||||||||
| ID (39%) | I.AD Mean | 2& | Grade 3 | |||||||||
| (Gy) | (1.5–4.0) | Esophagitis 4% | ||||||||||
| RCA Mean | 0.8 | Late: | ||||||||||
| (Gy) | (0.4–2.7) | Roconst. Cx - 39% | ||||||||||
| MSK (113) | 2013–2015 | 42 | LCW(n=36) | Metric | UP | Metric | UP | OAR | UP | 35 mo | Acute: | 3-year |
| R CW (n=6) | (Median) | (Range) | (Median) | (Range) | Metric | I Range) | Grade 2 | LR DFS 96.3% | ||||
| ♦IMN76% | Mean (Gy) | 0.7 | V20 (%) | 7.1 | C/1 Breast | 7.1 | Dermatitis 74% | MFS 84.1% | ||||
| 0.0–3.2 | (0.1–19.1) | V5(%) | (0.0–9.9) | Esophagitis 17% | «5 97.2% | |||||||
| V2©(%) | 03 | IpdV20(%) | 16.1 | C/1 Breast | 03 | Skin Pain 24% | LC 97.6% | |||||
| 0.0–6.0 | (2.1–303) | Mean (Gy) | (OjO-33) | Fatigue 2% | ||||||||
| UP | — | Malian 50 Gy | V5(%) | 43 | Ipd V5(%) | 34.0 | Spinal Cord | 0.8 | Late: | |||
| +/−Boost | 0–162 | (16,4–53.8) | Max (Gy) | (0j0-I8.2) | Roconst.Cx −27% | |||||||
| Max (Gy) | 163 | C/1 V5 (%) | 03 | Esophagus | 73 | Grade 3 | ||||||
| 0.1–51.9 | (0–42.4) | Mean (Gy) | (OjO-26.9) | Pneumonitis: 2% | ||||||||
| NWM (114) | 2011–2016 | 91 | WB (n=27) | Constraint Ised | Constraint Used | Constrakit | 153 mo | Grade 2 | OS 93.4% | |||
| CW (n=66) | Used | Dermatitis 72% | DF 11% | |||||||||
| L(n=56) | V20 | £21% | Ipsi V20 | <33% | Esophagus Max | <40Gy | Esophagitis 33% | LC 95.6% | ||||
| R (n=33) | Ipsi Humeral | <45 Gy | SkinPain21% | |||||||||
| UP (n=72) | Tl-2 (n=55) | Median 50.4 Gy | V5 | £50% (Left-sided) | Ipd V5 | <42% | Head Max | Fatigue 15% | ||||
| PBS (n=21) | T3–4(n=38) | +/− 8.0–19.8 | <40% (Right-sidod) | Skin PTVVII0 | <33% | Grade 3 | ||||||
| N+ (n=83) | Gy Boo a | Dermatitis 5% | ||||||||||
| Series | Year / Modality | N / Stage | Target / Dose | Heart | Lung | Other Metric | Median F/u Toxicity Disease Control | |||||
| Other | ||||||||||||
| Rib Fracture - 3% | ||||||||||||
| Lymphedema - 3% | ||||||||||||
| UF (25) | 2012–2014 | 18 | WB (n=7) CW (n=l 1) L (N=9) | Metric (Median; L, R) | 3D | PP | Metric (Median) |
3D PP | OAR Metric | 3D | PP 20 mo Grade 2 Pneumonitis 6% Grade 3 |
|
| R (N=9) | V5 (%) | 34.0 13.2 |
2.7%# 0.3 |
Ipsi V5 (%) | 60.5 35.3 | Integral Dose (Gy-L) | 193 | 92.7 Dermatitis 22% Infection 6% | ||||
| Mean (Gy) | 5.9 2.9 |
0.6 >0.5 |
Ipsi V20 (%) | 35.5 21.6 | C7I Breast V3(%) |
0 | 0 | |||||
| PP(n=10) | IIA-IIIB | 50.4 Gy | V20 (%) | 6.1 | 1.0 | Ipsi Mean (Gy) | 17.5 11.0 | |||||
| PP/3D(N=8) | Br/CW + LN | 1.6 | 0.0 | |||||||||
| 3D | +/− 10–16Gy Boost |
Ventricle Mean (Gy) LAD Max (Gy) LAD Mean (Gy) |
5.4 1.2 44.6 27.0 |
0.1 0.2 30.5 1.7 |
||||||||
| MGH (112) | 12 | L CW (n=l 1) R CW (n=l) | Metric (Mean, L) Mean (Gy) |
PP (Range) 0.4 (0.1–1.2) |
Metric (Mean,) Mean (Gy) | PP (Range) 6 (2.4–10.1) |
6 mo Acute: Grade > 1 — 0% |
|||||
| PP | Tl-2 (n=9) T3 (n=3) N+ (n=l 1) | 50Gy CW 45–50.4 Gy LN | V20 (%) LV Mean (Gy) LV V20 (%) |
0.01 (0.0–2.4) 0.1 (0.0–0.4) 0.0004 (0.0–0.2) |
V20 (%) | 12.7 (4.4–22.1) |
||||||
Abbreviations: VM = volumetric modulated arc therapy: PP = passively scattered proton therapy: WT = 3D conformal wide tangents; IM = intensity modulated radiation therapy: PBS = pencil beam scanning proton therapy: UP = single field uniform proton therapy; 3D-CRT = 3D con formal radiotherapy: WB = whole breast; CW = chest wall, 1MN = internal mammary nodes; Ax = axilla: HI = homogeneity index; C/1 = contralateral; DIB H = deep inspiratory breast hold: Gy = Gray (Relative Biological Effectiveness [ RBE)): IQR = interquartile range: LAD = left anterior descending artery; LV = left ventricle; N = number of patients: mo = months: OS = overall survival; LC = local control; DF = distant failure: MFS = metastasis-free survival; LR DFS = local recurrence disease free survival; Rceonst. Cx = reconstruction complications; Br. Plcxop. = brachial plcxopathy; ROM = range of motion.
Bolded and italicized values have an associated p-value less than 0.05, indicating a statistically significant difference versus the comparative group, when these data were available
In a publication from the Procure Proton Therapy Center in New Jersey, 42 patients with breast cancer received adjuvant chest wall and RNI using single field uniform dose optimized PT113. Dosimetric parameters to the heart, lungs, contralateral breast, spinal cord, and esophagus were low, consistent with those seen in previous planning studies. With a median follow-up of 35 months, no grade 3 or higher acute toxicities were noted and only 1 (2%) grade 3 late complication, in the form of pneumonitis, developed in a patient who had prior contralateral RNI and a stem cell transplant. Three-year outcomes included 97.2% overall survival and 97.6% local control.
Retrospective outcomes of 91 patients treated with uniform scanning or pencil beam scanning (PBS) PT at Northwestern Medicine Chicago Proton Therapy Center have been described 114. Grade 2 or higher toxicities were limited, with 5% developing grade 3 esophagitis, rib fracture in 3%, and clinically evident lymphedema in 3%. With a median follow-up of 16 months, twelve patients experienced disease failure, and the crude locoregional control was 96%.
A prospective trial with long-term follow-up of breast cancer patients undergoing RNI was recently published41. Between 2011 and 2016, 69 patients were treated with passive scattering PT (prior to 2013) or PBS PT (2013 and later). 93% of patients were treated post-mastectomy41. The heart mean, LAD max, and ipsilateral lung V20 Gy were 0.5 Gy, 4.7 Gy (RBE), and 14.5%, respectively43. With a median follow-up of 55 months, 5-year locoregional control and overall survival rates were 98.5% and 91%, respectively41. Acute toxicities included grade 2 radiation dermatitis and fatigue in 83% and 35% of patients, respectively, and grade 3 radiation dermatitis in 4%. The acute dermatologic adverse events were comparable to what has recently been reported with conventionally fractionated photon patients40. Five patients (7%) experienced grade 1 rib fracture, a higher rate than other PT and photon experiences reported to date. This may be due to close attention due to a perceived potential increased risk with low threshold for imaging, use of a single field, and lack of chest wall constraint to limit end of range LET/RBE effects during the treatment planning process43. Research is ongoing that is examining potential differences between photons and protons with respect to chest wall toxicity (NCT03270072).
The pragmatic randomized phase III proton versus photon breast trial for RNI in nonmetastatic breast cancer patients (RADCOMP, RTOG 3510) is currently accruing (NCT02603341)69. The primary objective of this study is to assess the effectiveness of proton versus photon therapy in reducing MCEs. The hypothesis of the trial is that PT will reduce the 10-year rate of MCEs after radiation from 6.3% to 3.5%, a relative reduction of 45%. The predicted 6.3% 10-year event rate in the photon arm was estimated from the Surveillance Epidemiological End Results (SEER) database and is higher than that reported in the study by Darby and colleagues69. Using the 7.4% estimated relative increase in MCEs per gray mean heart dose from Darby et al., a 45% relative reduction would require an absolute difference in mean heart dose between the photon and proton arms of > 6 Gy12. Modern photon irradiation has seen improvements in cardiac sparing. For example, a mean heart dose > 5 Gy in the RNI arm of the ongoing NRG Oncology/NSABP B-51/RTOG 1304 trial would be a protocol violation. The heart doses observed in the photon arm, along with the age and comorbidities of the participants, may ultimately determine whether the RADCOMP trial is adequately powered to detect a clinically meaningful reduction in MCEs with PT at 10 years6. Secondary objectives of the RADCOMP trial include to assess the non-inferiority of proton versus photon therapy in reducing locoregional and any recurrence, to assess health-related quality of life (HRQOL) and adverse events, and to develop predictive models to examine the association of radiation dose distribution to heart and MCEs and HRQOL. The Danish Breast Cancer Group is also conducting a phase III randomized breast cancer trial. In this study eligible patients are those with indications for adjuvant radiotherapy for breast cancer where standard photon planning shows a mean heart dose of 4 Gy or more and/or an ipsilateral lung V20 Gy of 37% or more117. The primary endpoint is radiation associated ischemic and valvular heart disease at 10 years after radiotherapy. Much larger studies will be required to test the hypothesis that the improved CTV coverage observed with PT can reduce breast cancer recurrence events over modern photon-based techniques6,63. The optimal dose/fractionation for PT in patients undergoing RNI is not known. Hypofractionation is an area of ongoing investigation, including a randomized phase 2 trial investigating conventional versus moderate hypofractionation in patients undergoing PMRT (NCT02783690)37,115,116.
Reconstruction
Recommendation: PT is a treatment option for patients who have undergone a mastectomy with immediate breast reconstruction and have indications for PMRT. Clinical trial enrollment should be considered if available (LE: 3, Grade C).
Rates of mastectomy, contralateral prophylactic mastectomy (CPM), and immediate breast reconstruction are increasing 118–120. Reconstructed breast mounds may preclude comprehensive coverage of the postmastectomy CTV with photon techniques121. For example, among women who undergo CPM with direct-to-implant or tissue expander reconstruction, the breast prosthesis often retains a medial chest wall position, even when the patient is supine. This medial positioning may result in wide tangent or IMRT fields traversing the contralateral prosthesis, particularly when the IMNs are targeted. An en face electron strip can limit contralateral chest wall dose, but differences in chest wall tissue thickness created by the reconstructed breast may limit chest wall and IMN CTV coverage31,121. Typically delivered with one or more en face fields, PT improves CTV coverage and sparing of the contralateral breast mound and other organs at risk in reconstructed women2,31,99. Another potential advance of PT among patients undergoing autologous flap reconstruction is improved sparing of the region of the internal mammary vessel anastomosis122,123.
PT clinical outcomes have been reported in women following mastectomy with IBR25,99,112. Smith et al. described outcomes of 51 reconstructed patients treated with multi-field optimized PBS PT (intensity modulated PT [IMPT]) at the Mayo Clinic 37. As expected, PT increased the risk of implant-based reconstruction complications compared with non-irradiated contralateral reconstructed breasts, but acute toxicity and reconstruction outcomes compared favorably with previously published photon studies124. These results were promising given the exceptional normal tissue sparing and target coverage afforded by PT, with a median mean heart, ipsilateral lung V20 Gy (RBE) and IMN CTV V95% of 0.6 Gy (RBE), 13.9%, and 97.4%, respectively37. Interestingly, the rate of reconstruction complications was higher in those receiving a moderately hypofractionated PT regimen (40.05 Gy [RBE] in 15 fractions) compared with conventional fractionation, although this subset analysis was limited by small patient numbers and could be a chance finding. Nevertheless, caution may be warranted when extrapolating novel fractionation regimens to PT based on photon experiences alone due to potential LET/RBE differences between the modalities, and additional investigation into altered dose fractionation regimens is warranted.
The aforementioned phase II trial from MGH included 39 patients who underwent mastectomy with immediate implant-based reconstruction and 14 patients who underwent delayed reconstruction41. Among this cohort of 53 patients who attempted reconstruction, 15 (28%) experienced an RT-related complication and just two (4%) experienced reconstructive loss. These outcomes compared favorably with recent reports of patients treated with photon therapy from that institution125.
Partial breast irradiation
Recommendation: PT is a treatment option for patients with indications for PBI. Clinical trial enrollment should be considered if available (LE: 3, Grade B).
Accelerated partial breast irradiation (APBI) has emerged as a standard of care for selected patients with favorable early stage breast cancer126–129. Favorable treatment outcomes have been achieved with modern short course photon therapy and brachytherapy126,128. Numerous studies have demonstrated that PT reduces non-target breast, heart, and lung exposure compared with photon techniques, and reduces heart and lung exposure and dose inhomogeneity compared to brachytherapy APBI94,130–134. Normal tissue sparing may be most pronounced in patients with medially located tumors. Reducing dose to non-target breast tissue with photon therapy has been associated with improved cosmesis following APBI135.
Outcomes from several prospective proton APBI studies have been reported (Table 3). Investigators at Loma Linda conducted a phase II trial of 100 patients treated with a multi-field, prone, passive scattered technique to a dose of 40 Gy (RBE) in 10 daily fractions. With a median follow-up of 5 years, the in-breast recurrence free-survival was 97%, and there were no grade 3 or higher acute skin toxicities. Late toxicity consisted of just 7 cases of grade I telangiectasia. Provider assessed cosmesis was good to excellent in over 90% of patients in annual follow-up through 5 years; patient-reported cosmesis was comparably favorable, with no evidence of deterioration over time 39,136. Comparably favorable three-year outcomes have also been presented from a multi-center phase II Proton Collaborative Group study utilizing the same dose-fractionation regimen137.
Table 3.
Clinical investigations of proton therapy for partial breast irradiation (PBI)
| Institution | Technique (N) | Dose | RT Details | MD-Reported Cosmesis | Patient-Reported Cosmesis |
Toxicity | Disease Control |
|---|---|---|---|---|---|---|---|
|
| |||||||
| MGH (38,96) | PP (n=19) | 32 Gy (RBE)/4 Gy x | 1–3 fields for PP (1 | 7yr Good-excellent | 7yr Good-excellent | PPvs. EBorP/E | 7yr LF: |
| EB or P/E (n=79) | 8 fx BID | field treated per fx) 1.5–2.0 cm margin to PTV <5 mm from skin |
62% PP 94% EB or P/E | 92% PP 96% photon, NSS |
Telangicctasias 69% vs. 16% (¿=0.03) Pigmentation changes 54% vs. 22% </>=0.02) Other late skin tox 62% vs. 18% (>=0.029) |
11% PP 4% EBorP/E, NSS |
|
| NCC, Korea (140) | PP (n=30) | 30 Gy (RBE) / 6 Gy x 5fx QD | 1–2 fields PBT | 3yr Good-excellent 69% (−100% with 2- fieldplan) | Increased toxicity with single field plan | 3yr LF 0% 3yr DF 0% | |
| LLU (39,136) | PP(n=90) | 40 Gy (RBE)/4 Gy x 10 fx QD | 2–4 fields PP 1.0 cm tumor bed margin to CTV | 5yr Good-excellent 90% | 5yr Good-excellent 90% | Acute: Grl-2-RT dermatitis 62% No grade 3 AE Late: Gr 1 - telangiectasia 7% Fat necrosis 1% |
5yr IBTR-FS 97% 5yr OS 95% |
| PC G BRE007 | UP(n=37) | 40 Gy (RBE)/4 Gy | >3 fields (>2 treated | 3yr Good-excellent | BCTOS Score of 4: | Gr 2 — 7 events | 3yr LF 0% |
| (137) | PP(n=l) | (RBE) x lOfxQD | daily) for PP 1.5 cm margin to CTV | 100% | 13.2% (nipple appearance, breast shape, scar tissue) | >Gr 3 — none | 3yr DF 0% |
| MDACC (141) | PP (n=100) | 34 Gy (RBE)/ 3.4 Gy x lOfx BID | >2 fields (3 or 4 fields used for 80% of patients) | 2yr Good-excellent 84% | 2yr Good-excellent: 96% | Acute Gr2 - Breast pain (1%), Hyperpigmcntation (2%), Pruritus (1%), Dermatitis (12%) Late Gr 2 - Fatigue (5%), Hyperpigmcntation (1%), Dermatitis (2%) >Gr 3 — none |
2yr LF 0% 2yr OS 100% |
Abbreviations: FP = passively scattered proton therapy; EB = photon external beam radiotherapy: P/E = photons with electrons; UP = uniform scanning proton therapy; RT = radiotherapy, fx = fractions; Gy = Gray; RBE = Radiobiological effective dose; NSS=not statistically significant; yr ) year; Gr = grade; LF = local failure; DF=distant failure; IBTR-FS ipsilateral bieast tumor recurrence-free survival; OS = overall survi val
Long-term results from the first dose cohort of a phase I APBI trial conducted at MGH that included a small number of patients treated with PT are available. APBI was administered with either aperture and compensator-based, double scattering PT (n=19) or photon 3DCRT (n=79)38. The prescription was 32 Gy (RBE) in 8 fractions given twice daily. The heart mean and maximum dose, D5%, D10%, and D20% were all significantly less with PT. In addition, PT had a lower ipsilateral lung mean and maximum dose, D5%, D10%, and D20% and greater non target breast sparing. No significant difference in 7-year incidence of local failure or in patient reported cosmetic outcomes between the two arms was found. However, PT APBI was associated with significantly worse provider-reported late skin toxicity and adverse cosmesis, likely related to the use of a single proton field and insufficient time for repair between the large, twice daily fractions38,138,139. The authors favored two fields treated daily based on these results.
The National Cancer Center in Korea has reported long term results of a phase 2 trial of 30 patients, aged ≥ 40 years with primary tumors ≤ 3 cm and pathologically negative nodes, treated with aperture and compensator-based proton APBI140. The prescription was 30 Gy (RBE) in 5 daily fractions. A single field was used for the first 15 patients and a two-field technique for the remaining 15 patients to limit entrance skin dose. There have been no recurrences, with a median follow-up of 59 months. Physician-assessed cosmetic outcomes were good or excellent in 69% at 3 years but increased to 89% for patients treated with 2 fields; no late toxicities ≥ grade 2 were observed amongst the patients treated with two fields140.
Results of an interim analysis of a phase 2 trial from the MD Anderson Cancer Center of 100 patients with node negative early stage breast cancer and DCIS141. Patients were treated with a minimum of two passively scattered proton fields to achieve minimal beam overlap on the patient surface and the prescription was 34 Gy in 10 fractions delivered twice daily. With a median follow-up of 24 months there have been no local recurrences or acute or late toxicities grade 3 or higher. Cosmesis was reported as good or excellent at 12 months by 91% of patients and 94% of physicians141.
Early outcomes of 76 patients treated with PBS APBI at the Mayo Clinic have also been reported95. PBS is attractive due the capacity to constrain the dose at the skin surface, which may limit the risk of acute and late skin toxicity (Figure 3). In this study, a three fraction regimen of 7.3 Gy (RBE) per fraction was administered with a median of two multi-field optimized PBS beams. The median of the maximum dose to 1 cc of the skin volume (D1cc), defined as the first 3 mm beneath the body surface, was 89% of prescription. The median mean heart dose was 0.0 Gy (RBE) and the median volume of ipsilateral breast receiving 50% or more of the prescribed dose was 28%. With a median follow-up of 12 months there was no ≥grade 2 acute skin toxicity reported, and no grade ≥2 late adverse events to date95. Additional follow-up will be necessary to fully evaluate late effects and long-term cosmetic outcomes with this approach. In summary, given these prospective clinical outcomes demonstrating safety and efficacy of PT with the improved dosimetry over other APBI alternatives, PT is an attractive option for the delivery of APBI.
Whole breast irradiation
Recommendation: Photon whole breast irradiation can typically be administered with favorable normal tissue sparing. PT may be considered an option, particularly for patients with complex anatomy resulting in higher doses to organs at risk or necessitating compromises in target coverage. Clinical trial enrollment should be considered if available (LE: 3, Grade C)
Modern photon therapy typically delivers radiotherapy to the whole breast with excellent normal tissue sparing142–145. Nevertheless, dosimetric studies have demonstrated potential for improvements in target coverage and reductions in dose to the heart and lungs with PT, including complete or near complete cardiac sparing143,146,147. The absolute improvement in normal tissue sparing may be most pronounced in patients with complex anatomy such as pectus excavatum or pectus carinatum, medially located tumor beds, and in those unable to perform cardiac sparing maneuvers such as DIBH or prone positioning (Figure 5)148. The potential clinical importance of normal tissue sparing may be greater in patients with active cardiopulmonary conditions12,35.
Figure 5:
Sagittal (A) and axial (B) 20 Gy color wash images of a patient with locally advanced left breast cancer and pectus carinatum, a deformity of the chest characterized by protrusion of the sternum and ribs, demonstrating excellent coverage of the chest wall (pink) and IMN (red) CTV and normal tissue sparing despite the unfavorable anatomy.
Thorpe and colleagues recently reported early outcomes of 82 patients treated with whole breast irradiation with or without RNI, as part of the multi-institutional prospective Proton Collaborative Group (PCG) Registry149. Grade 3 adverse events were observed in 7% of patients, dermatitis and/or breast pain, and the authors concluded PT to be well tolerated. Similar to the case of APBI, skin-sparing PBS technology may make PT more attractive for whole breast irradiation95.
Bilateral Breast Cancer
Recommendation: PT is a treatment option for patients with indications for bilateral breast/chest wall irradiation. The benefit may be most pronounced when bilateral breast/chest wall with comprehensive RNI is indicated. Clinical trial enrollment should be considered if available (LE: 3, Grade C).
The incidence of synchronous bilateral breast cancer is 1–3 % of all patients affected by breast cancer150,151. Bilateral breast cancer radiotherapy increases the heart and lung dose when compared to unilateral treatment152 which puts patients at increased risk for cardiac and pulmonary adverse events5,20. In addition, uniform target coverage of the bilateral internal mammary chains and medial breast tissue is limited with traditional 3DCRT techniques due to overlap of the tangent fields. PT enables a homogeneous and highly conformal dose distribution with improved normal tissue sparing (Figure 2)153,154.
Reduced Arm Mobility
Recommendation: If available, PT may be considered a preferred treatment option for breast radiotherapy that must be delivered in the arms down position, particularly when RNI is indicated. Clinical trial enrollment should be considered if available (LE: 3, Grade C).
Patients with reduced arm mobility may require treatment in the arms down position. Photon tangents, delivered alone or in combination with matched electron fields, must be directed through the arm in such situations to achieve CTV coverage. Breast brachytherapy is an option but is not routinely utilized to treat the regional nodes. IMRT can improve arm sparing, but at the price of elevated heart and lungs doses from concentrated delivery of anteriorly directed fields or arcs to avoid the arm. In contrast, anterior PT fields are routinely administered, and achieve exquisite CTV coverage and normal tissue sparing in an arms down/akimbo position (Figure 2)93,99. Therefore, the dosimetric advantages of PT over photon techniques are particularly pronounced when breast radiotherapy must be administered in the arms down position155. The technique of arms down PT has been described, and favorable long-term disease control and toxicity outcomes have been reported37,41,93,99.
Reirradiation
Recommendation: PT is a treatment option for patients with indications for reirradiation. Clinical trial enrollment should be considered if available (LE: 3, Grade C).
Locoregional recurrence from breast cancer represents a broad spectrum of disease with differing natural history, but has generally been associated with poor prognosis156. The role and optimal approach to reirradiation, such as part of salvage treatment of recurrent breast cancer or in the management of second primary breast cancer or primary breast cancer following thoracic radiotherapy, has yet to be defined. Traditionally, options have included photon 3DCRT or IMRT, electrons, and brachytherapy157–162. In-field complications of reirradiation such as soft tissue fibrosis cannot be avoided with PT. However, when indicated, reirradiation may be made safer with PT by lowering cumulative doses to sensitive organs such as heart, lung, ribs, brachial plexus, and other soft tissue outside of the target volume or reducing dose heterogeneity from brachytherapy24,163.
Thorpe and colleagues have reported outcomes of 50 patients who in a multi-institutional prospective registry underwent PT reirradiation between 2011 and 2016164. The median time between RT courses and cumulative dose was 103.8 months and 100.6 Gy (RBE), respectively. With a median follow-up of 13 months from reirradiation, any (acute of late) grade 3 adverse events were observed in 16% of patients, including 4 patients (8%) with late grade 3 toxicities. Grade 3 adverse events were significantly associated with body mass index, bilateral recurrence, and bilateral reirradiation. One-year locoregional recurrence free and overall survival was 93% and 97%, respectively. These promising outcomes mirror prospective PT series in other disease sites165–167. Fattahi et al. reported outcomes of a series of 72 patients who underwent reirradiation between 1999 and 2019 at a single institution, including 52 patients treated with photons and 20 with protons. Any grade 3 adverse events were observed in 13% of patients, including 10% with acute skin toxicity. Two patients (3%) had grade 3 late adverse events, both treated with photon therapy168. These proton outcomes are comparable to prior photon reports169. Given the favorable dosimetric profile and early outcomes, PT is an attractive option for breast cancer reirradiation and further clinical evaluation is warranted.
COST EFFECTIVENESS AND MODEL-BASED PATIENT SELECTION
Due to the increased capital investment required to build and operate proton facilities, the cost of PT per fraction is greater than conventional photon radiotherapy. In addition, depending on the geographic location of the patient relative to PT centers, there may be additional unique costs related to travel, lodging, and time away from work. In the absence of phase III data, decision-analysis methodology and cost-effectiveness analyses (CEAs) can leverage known data to provide decision-makers with evidence to improve management. As such, different CEAs have been published examining the cost-effectiveness of PT170–173. For example, Mailhot Vega et. al published a contemporary CEA using an American framework informed by cardiac dose-toxicity relationships presented by Darby et al.9 Guidelines estimating mean heart dose thresholds for photon plans above which PT may be cost-effective comparatively were presented173. In the base case, it was assumed that cost of cardiac toxicity management would be exclusive medical management, an assumption that may severely underestimate the true cost of heart disease. Nevertheless, PT favorability mean heart dose thresholds approximating 7–9 Gy (RBE) for women without cardiac risk factors and 4–5 Gy (RBE) for women with at least one cardiac risk factor were calculated. In light of a systematic review of mean heart doses for photon-based regional nodal radiation20, the authors noted that PT may be cost-effective for more than 95% of women with ≥1 cardiac risk factor undergoing RNI for left breast cancer.
As an alternative to randomized controlled clinical trials comparing photons and protons, which the authors note may be impractical in many scenarios given the rapidly evolving technology and prolonged latency of radiation-associated late effects, Langendijk et al. have proposed and implemented a two phase model-based approach for patient selection for PT in the Netherlands9. The first phase aims to select patients who may benefit most from PT by integrating comparative planning studies with normal tissue complication probability (NTCP) models, and the second phase involves prospective observational cohort studies using historical comparisons as validation. The implemented model uses the calculated life-time risk for a cardiovascular event according to the publication by Darby et al12. The threshold for reimbursement of proton treatment is an absolute reduction of at least 2% in lifetime risk of a cardiovascular event.
Another area of exploration has been the cost favorability of proton APBI. While no CEAs with effectiveness endpoints have been captured, a cost analysis noted comparable cost of proton APBI versus other APBI techniques174. Hypofractionated and ultra-hypofractionated regimens have emerged as new standards in early stage breast cancer patients treated with photon therapy175–177. If also proven acceptable for PT, or if the improved normal tissue sparing of PT facilitates further treatment acceleration, the reduction in costs may further improve the cost-acceptability of PT95,178.
CONCLUSION
PT is being investigated as an adjuvant radiotherapy alternative for both early-stage and locally advanced breast cancer. Access to this modality has improved with the construction of PT centers throughout the globe. Long term clinical data will continue to become available in the years ahead, including the results from ongoing randomized trials, and these studies are expected to inform which patients are most likely to benefit from PT. Still, the field of PT for breast cancer remains in its infancy, and rapid technological advances are poised to further improve treatment delivery. These advances and potential variability in techniques across institutions also present challenges for evidence development. Close attention to the PT techniques employed is vital in evaluating available and burgeoning PT outcomes. Additional research is needed to fully explore the distinct physical and biological characteristics of PT179, including optimizing dose-fractionation schedules, in order to improve cure rates and optimize quality of life.
Acknowledgments
Data Sharing Statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Funding: This work was supported in part by K12 HD065987 (RWM)
Footnotes
Conflicts of Interest: Dr. Mailhot reports travel funds from Varian (2017) and IBA (2018); Dr. Bradley reports travel funds from IBA (2018); Dr. Ho reports no disclosures related to this submission. Unrelated to this manuscript, Dr. Ho has received research funding from Merck and GSK and received consulting fees from La Roche Posay; Dr. Fagundes reports being a consultant for Augmenix and Boston Scientific related to lecturing and training of new users of rectal spacers. Dr. Amos reports no disclosures related to this submission. Unrelated to this manuscript, Dr. Amos reports being on the scientific advisory board and receiving honoraria from TAE Life Sciences. Dr. Xuanfeng has a patent related to spot-scanning proton arc therapy and this patent has been licensed to IBA. The rest of the authors report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontanilla HP, Woodward WA, Lindberg ME, et al. Current clinical coverage of Radiation Therapy Oncology Group-defined target volumes for postmastectomy radiation therapy. Pract Radiat Oncol 2012;2:201–9. [DOI] [PubMed] [Google Scholar]
- 3.Thorsen LB, Thomsen MS, Berg M, et al. CT-planned internal mammary node radiotherapy in the DBCG-IMN study: benefit versus potentially harmful effects. Acta oncologica 2014;53:1027–34. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald SM, Harisinghani MG, Katkar A, Napolitano B, Wolfgang J, Taghian AG. Nanoparticle-enhanced MRI to evaluate radiation delivery to the regional lymphatics for patients with breast cancer. Int J Radiat Oncol Biol Phys 2010;77:1098–104. [DOI] [PubMed] [Google Scholar]
- 5.Taylor C, Correa C, Duane FK, et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J Clin Oncol 2017;35:1641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbin KS, Mutter RW. Proton therapy for breast cancer: progress & pitfalls. Breast Cancer Manag 2018;7. [Google Scholar]
- 7.Glatstein E, Glick J, Kaiser L, Hahn SM. Should randomized clinical trials be required for proton radiotherapy? An alternative view. J Clin Oncol 2008;26:2438–9. [DOI] [PubMed] [Google Scholar]
- 8.Goitein M, Cox JD. Should randomized clinical trials be required for proton radiotherapy? J Clin Oncol 2008;26:175–6. [DOI] [PubMed] [Google Scholar]
- 9.Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol 2013;107:267–73. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Research Institute C, Translational Radiotherapy Research Working Group Proton Beam Clinical Trial Strategy G. Proton Beam Therapy - the Challenges of Delivering High-quality Evidence of Clinical Benefit. Clin Oncol (R Coll Radiol) 2018;30:280–4. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice Guidelines ea. Clinical Practice Guidelines We can Trust. National Academies Press (US)2011. [Google Scholar]
- 12.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 13.van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients With Breast Cancer Treated With Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J Clin Oncol 2017;35:1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor C, McGale P, Bronnum D, et al. Cardiac Structure Injury After Radiotherapy for Breast Cancer: Cross-Sectional Study With Individual Patient Data. J Clin Oncol 2018;36:2288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wennstig AK, Garmo H, Isacsson U, et al. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat Oncol 2019;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuohinen SS, Skytta T, Virtanen V, Luukkaala T, Kellokumpu-Lehtinen PL, Raatikainen P. Early effects of adjuvant breast cancer radiotherapy on right ventricular systolic and diastolic function. Anticancer Res 2015;35:2141–7. [PubMed] [Google Scholar]
- 17.Saiki H, Petersen IA, Scott CG, et al. Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation 2017;135:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.. Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med 1999;341:226–32. [DOI] [PubMed] [Google Scholar]
- 19.. Leon-Ferre RA, Giridhar KV, Hieken TJ, et al. A contemporary review of male breast cancer: current evidence and unanswered questions. Cancer Metast Rev 2018;37:599–614. [DOI] [PubMed] [Google Scholar]
- 20.Taylor CW, Wang Z, Macaulay E, Jagsi R, Duane F, Darby SC. Exposure of the Heart in Breast Cancer Radiation Therapy: A Systematic Review of Heart Doses Published During 2003 to 2013. Int J Radiat Oncol Biol Phys 2015;93:845–53. [DOI] [PubMed] [Google Scholar]
- 21.Loap P, Kirova Y. Evaluating cardiac substructure radiation exposure in breast rotational intensity modulated radiation therapy: Effects of cancer laterality, fractionation and deep inspiration breath-hold. Cancer Radiother 2021;25:13–20. [DOI] [PubMed] [Google Scholar]
- 22.Zellars R, Bravo PE, Tryggestad E, et al. SPECT analysis of cardiac perfusion changes after whole-breast/chest wall radiation therapy with or without active breathing coordinator: results of a randomized phase 3 trial. Int J Radiat Oncol Biol Phys 2014;88:778–85. [DOI] [PubMed] [Google Scholar]
- 23.Lo Q, Hee L, Batumalai V, et al. Subclinical cardiac dysfunction detected by strain imaging during breast irradiation with persistent changes 6 weeks after treatment. Int J Radiat Oncol Biol Phys 2015;92:268–76. [DOI] [PubMed] [Google Scholar]
- 24.Ranger A, Dunlop A, Hutchinson K, et al. A Dosimetric Comparison of Breast Radiotherapy Techniques to Treat Locoregional Lymph Nodes Including the Internal Mammary Chain. Clin Oncol (R Coll Radiol) 2018;30:346–53. [DOI] [PubMed] [Google Scholar]
- 25.Bradley JA, Dagan R, Ho MW, et al. Initial Report of a Prospective Dosimetric and Clinical Feasibility Trial Demonstrates the Potential of Protons to Increase the Therapeutic Ratio in Breast Cancer Compared With Photons. Int J Radiat Oncol Biol Phys 2016;95:411–21. [DOI] [PubMed] [Google Scholar]
- 26.Cuaron JJ, Chon B, Tsai H, et al. Early toxicity in patients treated with postoperative proton therapy for locally advanced breast cancer. Int J Radiat Oncol Biol Phys 2015;92:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ares C, Khan S, Macartain AM, et al. Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys 2010;76:685–97. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez M ZR, Sanders M, et al. A treatment planning comparison of volumetric modulated arc therapy and proton therapy for a sample of breast cancer patients treated with postmastectomy radiotherapy. J Proton Ther 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flejmer AM, Edvardsson A, Dohlmar F, et al. Respiratory gating for proton beam scanning versus photon 3D-CRT for breast cancer radiotherapy. Acta Oncol 2016;55:577–83. [DOI] [PubMed] [Google Scholar]
- 30.Fagundes MH EB; Pankuch M et al. Proton therapy for local-regionally advanced breast cancer maximized cardiac sparing. Int J Particle Ther 2015;1:827–44. [Google Scholar]
- 31.Jimenez RB, Goma C, Nyamwanda J, et al. Intensity modulated proton therapy for postmastectomy radiation of bilateral implant reconstructed breasts: a treatment planning study. Radiother Oncol 2013;107:213–7. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald SM, Jimenez R, Paetzold P, et al. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiation oncology 2013;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gokula K, Earnest A, Wong LC. Meta-analysis of incidence of early lung toxicity in 3-dimensional conformal irradiation of breast carcinomas. Radiat Oncol 2013;8:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman CD, Nijman SFM, Senan S, et al. A Primer on Interstitial Lung Disease and Thoracic Radiation. J Thorac Oncol 2020;15:902–13. [DOI] [PubMed] [Google Scholar]
- 36.Giaj-Levra N, Sciascia S, Fiorentino A, et al. Radiotherapy in patients with connective tissue diseases. Lancet Oncol 2016;17:e109–e17. [DOI] [PubMed] [Google Scholar]
- 37.Smith NL, Jethwa KR, Viehman JK, et al. Post-mastectomy intensity modulated proton therapy after immediate breast reconstruction: Initial report of reconstruction outcomes and predictors of complications. Radiother Oncol 2019;140:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galland-Girodet S, Pashtan I, MacDonald SM, et al. Long-term Cosmetic Outcomes and Toxicities of Proton Beam Therapy Compared With Photon-Based 3-Dimensional Conformal Accelerated Partial-Breast Irradiation: A Phase 1 Trial. Int J Radiat Oncol Biol Phys 2014;90:493–500. [DOI] [PubMed] [Google Scholar]
- 39.Bush DA, Do S, Lum S, et al. Partial breast radiation therapy with proton beam: 5-year results with cosmetic outcomes. Int J Radiat Oncol Biol Phys 2014;90:501–5. [DOI] [PubMed] [Google Scholar]
- 40.Whelan TJ, Olivotto IA, Levine MN. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:1878–9. [DOI] [PubMed] [Google Scholar]
- 41.Jimenez RB, Hickey S, DePauw N, et al. Phase II Study of Proton Beam Radiation Therapy for Patients With Breast Cancer Requiring Regional Nodal Irradiation. J Clin Oncol 2019;37:2778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Underwood TSA, Grassberger C, Bass R, et al. Asymptomatic Late-phase Radiographic Changes Among Chest-Wall Patients Are Associated With a Proton RBE Exceeding 1.1. Int J Radiat Oncol Biol Phys 2018;101:809–19. [DOI] [PubMed] [Google Scholar]
- 43.Mutter RW, Jethwa KR, Wan Chan Tseung HS, et al. Incorporation of Biologic Response Variance Modeling Into the Clinic: Limiting Risk of Brachial Plexopathy and Other Late Effects of Breast Cancer Proton Beam Therapy. Pract Radiat Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang CC, McNamara AL, Shin J, et al. End-of-Range Radiobiological Effect on Rib Fractures in Patients Receiving Proton Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys 2020;107:449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grantzau T, Thomsen MS, Vaeth M, Overgaard J. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol 2014;111:366–73. [DOI] [PubMed] [Google Scholar]
- 46.Paganetti H, Depauw N, Johnson A, Forman RB, Lau J, Jimenez R. The risk for developing a secondary cancer after breast radiation therapy: Comparison of photon and proton techniques. Radiother Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leonardis JM, Diefenbach BJ, Lyons DA, et al. The influence of reconstruction choice and inclusion of radiation therapy on functional shoulder biomechanics in women undergoing mastectomy for breast cancer. Breast Cancer Res Treat 2019;173:447–53. [DOI] [PubMed] [Google Scholar]
- 48.Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv 2017;11:791–9. [DOI] [PubMed] [Google Scholar]
- 49.Bentzen SM, Overgaard M, Thames HD. Fractionation sensitivity of a functional endpoint: impaired shoulder movement after post-mastectomy radiotherapy. Int J Radiat Oncol Biol Phys 1989;17:531–7. [DOI] [PubMed] [Google Scholar]
- 50.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farace P, Deidda MA, Amichetti M. Axillary irradiation omitting axillary dissection in breast cancer: is there a role for shoulder-sparing proton therapy? Br J Radiol 2015;88:20150274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stovall M, Smith SA, Langholz BM, et al. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys 2008;72:1021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hooning MJ, Aleman BM, Hauptmann M, et al. Roles of radiotherapy and chemotherapy in the development of contralateral breast cancer. J Clin Oncol 2008;26:5561–8. [DOI] [PubMed] [Google Scholar]
- 54.Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol 2006;24:2437–43. [DOI] [PubMed] [Google Scholar]
- 55.Kirova YM, Savignoni A, Sigal-Zafrani B, et al. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat 2010;120:119–26. [DOI] [PubMed] [Google Scholar]
- 56.Bergom C, West CM, Higginson DS, et al. The Implications of Genetic Testing on Radiation Therapy Decisions: A Guide for Radiation Oncologists. Int J Radiat Oncol Biol Phys 2019;105:698–712. [DOI] [PubMed] [Google Scholar]
- 57.Settatree S, Brand D, Ranger A, et al. Estimating Contralateral Breast Cancer Risk from Photons versus Protons in Patients Undergoing Internal Mammary Nodal Breast Cancer Radiotherapy. Clin Oncol (R Coll Radiol) 2020;32:342. [DOI] [PubMed] [Google Scholar]
- 58.Arsene-Henry A, Foy JP, Robilliard M, et al. The use of helical tomotherapy in the treatment of early stage breast cancer: indications, tolerance, efficacy-a single center experience. Oncotarget 2018;9:23608–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–106. [DOI] [PubMed] [Google Scholar]
- 60.Xiang M, Chang DT, Pollom EL. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 2020;126:3560–8. [DOI] [PubMed] [Google Scholar]
- 61.Sethi RV, Shih HA, Yeap BY, et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer 2014;120:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang JS, Byun HK, Kim JW, et al. Three-dimensional analysis of patterns of locoregional recurrence after treatment in breast cancer patients: Validation of the ESTRO consensus guideline on target volume. Radiother Oncol 2017;122:24–9. [DOI] [PubMed] [Google Scholar]
- 63.Thorsen LB, Offersen BV, Dano H, et al. DBCG-IMN: A Population-Based Cohort Study on the Effect of Internal Mammary Node Irradiation in Early Node-Positive Breast Cancer. J Clin Oncol 2016;34:314–20. [DOI] [PubMed] [Google Scholar]
- 64.Brown LC, Diehn FE, Boughey JC, et al. Delineation of Supraclavicular Target Volumes in Breast Cancer Radiation Therapy. In Reply to Yang and Guo. Int J Radiat Oncol Biol Phys 2015;93:723–4. [DOI] [PubMed] [Google Scholar]
- 65.Jing H, Wang SL, Li J, et al. Mapping Patterns of Ipsilateral Supraclavicular Nodal Metastases in Breast Cancer: Rethinking the Clinical Target Volume for High-risk Patients. Int J Radiat Oncol Biol Phys 2015;93:268–76. [DOI] [PubMed] [Google Scholar]
- 66.Brown LC, Diehn FE, Boughey JC, et al. Delineation of Supraclavicular Target Volumes in Breast Cancer Radiation Therapy. Int J Radiat Oncol Biol Phys 2015;92:642–9. [DOI] [PubMed] [Google Scholar]
- 67.DeSelm C, Yang TJ, Cahlon O, et al. A 3-Dimensional Mapping Analysis of Regional Nodal Recurrences in Breast Cancer. Int J Radiat Oncol Biol Phys 2019;103:583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borm KJ, Voppichler J, Dusberg M, et al. FDG/PET-CT-Based Lymph Node Atlas in Breast Cancer Patients. Int J Radiat Oncol Biol Phys 2019;103:574–82. [DOI] [PubMed] [Google Scholar]
- 69.Bekelman JE, Lu H, Pugh S, et al. Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: the Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol. BMJ Open 2019;9:e025556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 2015;114:3–10. [DOI] [PubMed] [Google Scholar]
- 71.Vargo JA, Beriwal S. RTOG Chest Wall Contouring Guidelines for Post-Mastectomy Radiation Therapy: Is It Evidence-Based? Int J Radiat Oncol Biol Phys 2015;93:266–7. [DOI] [PubMed] [Google Scholar]
- 72.Kaidar-Person O, Vrou Offersen B, Hol S, et al. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol 2019;137:159–66. [DOI] [PubMed] [Google Scholar]
- 73.Langstein HN, Cheng MH, Singletary SE, et al. Breast cancer recurrence after immediate reconstruction: patterns and significance. Plast Reconstr Surg 2003;111:712–20; discussion 21–2. [DOI] [PubMed] [Google Scholar]
- 74.Sbitany H, Gomez-Sanchez C, Piper M, Lentz R. Prepectoral Breast Reconstruction in the Setting of Postmastectomy Radiation Therapy: An Assessment of Clinical Outcomes and Benefits. Plast Reconstr Surg 2019;143:10–20. [DOI] [PubMed] [Google Scholar]
- 75.Schaeffer CV, Dassoulas KR, Thuman J, Campbell CA. Early Functional Outcomes After Prepectoral Breast Reconstruction: A Case-Matched Cohort Study. Ann Plast Surg 2019;82:S399–S403. [DOI] [PubMed] [Google Scholar]
- 76.Mutter RW. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol 2019;141:329–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paganetti H, Blakely E, Carabe-Fernandez A, et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med Phys 2019;46:e53–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oden J, Toma-Dasu I, Eriksson K, Flejmer AM, Dasu A. The influence of breathing motion and a variable relative biological effectiveness in proton therapy of left-sided breast cancer. Acta Oncol 2017;56:1428–36. [DOI] [PubMed] [Google Scholar]
- 79.Guan F, Geng CR, Ma D, et al. RBE Model-Based Biological Dose Optimization for Proton Radiobiology Studies. International Journal of Particle Therapy 2018;5:160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giovannini G, Bohlen T, Cabal G, et al. Variable RBE in proton therapy: comparison of different model predictions and their influence on clinical-like scenarios. Radiation Oncology 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Willers H, Allen A, Grosshans D, et al. Toward A variable RBE for proton beam therapy. Radiotherapy and Oncology 2018;128:68–75. [DOI] [PubMed] [Google Scholar]
- 82.Ding XF, Li XQ, Zhang JM, Kabolizadeh P, Stevens C, Yan D. Spot-Scanning Proton Arc (SPArc) Therapy: The First Robust and Delivery-Efficient Spot-Scanning Proton Arc Therapy. Int J Radiat Oncol 2016;96:1107–16. [DOI] [PubMed] [Google Scholar]
- 83.Moyers MF, Sardesai M, Sun S, Miller DW. Ion stopping powers and CT numbers. Med Dosim 2010;35:179–94. [DOI] [PubMed] [Google Scholar]
- 84.Paganetti H Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol 2012;57:R99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang M, Zhu XR, Park PC, et al. Comprehensive analysis of proton range uncertainties related to patient stopping-power-ratio estimation using the stoichiometric calibration. Phys Med Biol 2012;57:4095–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strom EA, Amos RA, Shaitelman SF, et al. Proton partial breast irradiation in the supine position: Treatment description and reproducibility of a multibeam technique. Pract Radiat Oncol 2015;5:e283–90. [DOI] [PubMed] [Google Scholar]
- 87.Liang X, Mailhot Vega RB, Li Z, Zheng D, Mendenhall N, Bradley JA. Dosimetric consequences of image guidance techniques on robust optimized intensity-modulated proton therapy for treatment of breast Cancer. Radiat Oncol 2020;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wiant DB, Wentworth S, Maurer JM, Vanderstraeten CL, Terrell JA, Sintay BJ. Surface imaging-based analysis of intrafraction motion for breast radiotherapy patients. J Appl Clin Med Phys 2014;15:4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cuaron JJ, MacDonald SM, Cahlon O. Novel applications of proton therapy in breast carcinoma. Chin Clin Oncol 2016;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bradley JA, Ho MW, Li Z, et al. A Technical Guide for Passive Scattering Proton Radiation Therapy for Breast Cancer. Int J Part Ther 2017;3:473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albertini F, Matter M, Nenoff L, Zhang Y, Lomax A. Online daily adaptive proton therapy. Br J Radiol 2020;93:20190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu RY, Liu AY, Sio TT, et al. Intensity-Modulated Proton Therapy Adaptive Planning for Patients with Oropharyngeal Cancer. Int J Part Ther 2017;4:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Depauw N, Batin E, Daartz J, et al. A novel approach to postmastectomy radiation therapy using scanned proton beams. Int J Radiat Oncol Biol Phys 2015;91:427–34. [DOI] [PubMed] [Google Scholar]
- 94.Bush DA, Slater JD, Garberoglio C, Yuh G, Hocko JM, Slater JM. A technique of partial breast irradiation utilizing proton beam radiotherapy: comparison with conformal x-ray therapy. Cancer J 2007;13:114–8. [DOI] [PubMed] [Google Scholar]
- 95.Mutter RW, Jethwa KR, Gonuguntla K, et al. 3 fraction pencil-beam scanning proton accelerated partial breast irradiation: early provider and patient reported outcomes of a novel regimen. Radiat Oncol 2019;14:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kozak KR, Smith BL, Adams J, et al. Accelerated partial-breast irradiation using proton beams: initial clinical experience. Int J Radiat Oncol Biol Phys 2006;66:691–8. [DOI] [PubMed] [Google Scholar]
- 97.Wang X, Amos RA, Zhang X, et al. External-beam accelerated partial breast irradiation using multiple proton beam configurations. Int J Radiat Oncol Biol Phys 2011;80:1464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amos RA, Woodward WA. Field match verification during combination proton, photon, and electron therapy for oligometastatic inflammatory breast cancer. Med Dosim 2012;37:442–4. [DOI] [PubMed] [Google Scholar]
- 99.Mutter RW, Remmes NB, Kahila MM, et al. Initial clinical experience of postmastectomy intensity modulated proton therapy in patients with breast expanders with metallic ports. Pract Radiat Oncol 2017;7:e243–e52. [DOI] [PubMed] [Google Scholar]
- 100.Jia Y, Zhao L, Cheng CW, McDonald MW, Das IJ. Dose perturbation effect of metallic spinal implants in proton beam therapy. J Appl Clin Med Phys 2015;16:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Axente M, Paidi A, Von Eyben R, et al. Clinical evaluation of the iterative metal artifact reduction algorithm for CT simulation in radiotherapy. Med Phys 2015;42:1170–83. [DOI] [PubMed] [Google Scholar]
- 102.Kirk M, Freedman G, Ostrander T, Dong L. Field-Specific Intensity-modulated Proton Therapy Optimization Technique for Breast Cancer Patients with Tissue Expanders Containing Metal Ports. Cureus 2017;9:e1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bergom C, Currey A, Desai N, Tai A, Strauss JB. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front Oncol 2018;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patel SA, Lu HM, Nyamwanda JA, et al. Postmastectomy radiation therapy technique and cardiopulmonary sparing: A dosimetric comparative analysis between photons and protons with free breathing versus deep inspiration breath hold. Pract Radiat Oncol 2017;7:e377–e84. [DOI] [PubMed] [Google Scholar]
- 105.Yu J, Park SS, Herman MG, Langen K, Mehta M, Feigenberg SJ. Free Breathing versus Breath-Hold Scanning Beam Proton Therapy and Cardiac Sparing in Breast Cancer. Int J Part Ther 2017;3:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949–55. [DOI] [PubMed] [Google Scholar]
- 107.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641–8. [DOI] [PubMed] [Google Scholar]
- 108.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997;337:956–62. [DOI] [PubMed] [Google Scholar]
- 109.Poortmans PM, Collette S, Kirkove C, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 2015;373:317–27. [DOI] [PubMed] [Google Scholar]
- 110.Jethwa KR, Kahila MM, Whitaker TJ, et al. Immediate tissue expander or implant-based breast reconstruction does not compromise the oncologic delivery of post-mastectomy radiotherapy (PMRT). Breast Cancer Res Treat 2017;164:237–44. [DOI] [PubMed] [Google Scholar]
- 111.Xiang M, Chang DT, Pollom EL. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 2020. [DOI] [PubMed] [Google Scholar]
- 112.Macdonald SM, Patel SA, Hickey S, et al. Proton therapy for breast cancer after mastectomy: early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys 2013;86:484–90. [DOI] [PubMed] [Google Scholar]
- 113.Luo L, Cuaron J, Braunstein L, et al. Early outcomes of breast cancer patients treated with postmastectomy uniform scanning proton therapy. Radiother Oncol 2018. [DOI] [PubMed] [Google Scholar]
- 114.Verma V, Iftekaruddin Z, Badar N, et al. Proton beam radiotherapy as part of comprehensive regional nodal irradiation for locally advanced breast cancer. Radiother Oncol 2017;123:294–8. [DOI] [PubMed] [Google Scholar]
- 115.Brunt AM. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brunt AM, Haviland JS, Sydenham M, et al. Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer. J Clin Oncol 2020;38:3261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stick LB, Lorenzen EL, Yates ES, et al. Selection criteria for early breast cancer patients in the DBCG proton trial - The randomised phase III trial strategy. Clin Transl Radiat Oncol 2021;27:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Panchal H, Pilewskie ML, Sheckter CC, et al. National trends in contralateral prophylactic mastectomy in women with locally advanced breast cancer. J Surg Oncol 2019;119:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dragun AE, Huang B, Tucker TC, Spanos WJ. Increasing mastectomy rates among all age groups for early stage breast cancer: a 10-year study of surgical choice. Breast J 2012;18:318–25. [DOI] [PubMed] [Google Scholar]
- 120.Portschy PR, Tuttle TM. Rise of mastectomy. J Surg Oncol 2013;107:563–4. [DOI] [PubMed] [Google Scholar]
- 121.Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:76–82. [DOI] [PubMed] [Google Scholar]
- 122.Amabile N, Veugeois A, Zannis K, Caussin C. Radiotherapy-induced vascular damage in mammary arterial graft: correlations between optical coherence tomography and pathology. Eur Heart J 2016;37:567. [DOI] [PubMed] [Google Scholar]
- 123.Cherubino MS S; Taibi D et al. Short-term effects of radiotherapy postquadrantectomy on internal mammary artery and vein. ISRN Plastic Surgery 2013;2013. [Google Scholar]
- 124.Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol 2014;21:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Naoum GE, Salama L, Niemierko A, et al. Single Stage Direct-to-Implant Breast Reconstruction Has Lower Complication Rates Than Tissue Expander and Implant and Comparable Rates to Autologous Reconstruction in Patients Receiving Postmastectomy Radiation. Int J Radiat Oncol Biol Phys 2020;106:514–24. [DOI] [PubMed] [Google Scholar]
- 126.Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet 2016;387:229–38. [DOI] [PubMed] [Google Scholar]
- 127.Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017;390:1048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 2015;51:451–63. [DOI] [PubMed] [Google Scholar]
- 129.Correa C, Harris EE, Leonardi MC, et al. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol 2017;7:73–9. [DOI] [PubMed] [Google Scholar]
- 130.Vicini FA, Remouchamps V, Wallace M, et al. Ongoing clinical experience utilizing 3D conformal external beam radiotherapy to deliver partial-breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 2003;57:1247–53. [DOI] [PubMed] [Google Scholar]
- 131.Taghian AG, Kozak KR, Katz A, et al. Accelerated partial breast irradiation using proton beams: Initial dosimetric experience. Int J Radiat Oncol Biol Phys 2006;65:1404–10. [DOI] [PubMed] [Google Scholar]
- 132.Kozak KR, Katz A, Adams J, et al. Dosimetric comparison of proton and photon three-dimensional, conformal, external beam accelerated partial breast irradiation techniques. Int J Radiat Oncol Biol Phys 2006;65:1572–8. [DOI] [PubMed] [Google Scholar]
- 133.Moon SH, Shin KH, Kim TH, et al. Dosimetric comparison of four different external beam partial breast irradiation techniques: three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, helical tomotherapy, and proton beam therapy. Radiother Oncol 2009;90:66–73. [DOI] [PubMed] [Google Scholar]
- 134.Hansen TM, Bartlett G, Mannina EM, Srivastava SP, Cox JA, Das IJ. Dosimetric Comparison of Treatment Techniques: Brachytherapy, IMRT, and Proton Beam in Partial-Breast Irradiation. Int J Radiat Oncol 2015;93:E8–E. [Google Scholar]
- 135.Jagsi R, Ben-David MA, Moran JM, et al. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys 2010;76:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bush DA, Slater JD, Garberoglio C, Do S, Lum S, Slater JM. Partial breast irradiation delivered with proton beam: results of a phase II trial. Clin Breast Cancer 2011;11:241–5. [DOI] [PubMed] [Google Scholar]
- 137.Choi IJ, Prabhu K, Hartsell WF, et al. Clinical Outcomes after Proton Partial-Breast Radiotherapy for Early-Stage, Hormone Receptor-Positive Breast Cancer: 3-Year Outcomes of a Phase II Trial. Int J Radiat Oncol 2019;105:E19–E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bentzen SM, Yarnold JR. Reports of unexpected late side effects of accelerated partial breast irradiation--radiobiological considerations. Int J Radiat Oncol Biol Phys 2010;77:969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Whelan TJ, Julian JA, Berrang TS, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet 2019;394:2165–72. [DOI] [PubMed] [Google Scholar]
- 140.Chang JH, Lee NK, Kim JY, et al. Phase II trial of proton beam accelerated partial breast irradiation in breast cancer. Radiother Oncol 2013;108:209–14. [DOI] [PubMed] [Google Scholar]
- 141.Pasalic D, Strom EA, Allen PK, et al. Proton Accelerated Partial Breast Irradiation: Clinical Outcomes at a Planned Interim Analysis of a Prospective Phase 2 Trial. Int J Radiat Oncol Biol Phys 2021;109:441–8. [DOI] [PubMed] [Google Scholar]
- 142.Bronsart E, Dureau S, Xu HP, et al. Whole breast radiotherapy in the lateral isocentric lateral decubitus position: Long-term efficacy and toxicity results. Radiother Oncol 2017;124:214–9. [DOI] [PubMed] [Google Scholar]
- 143.Mast ME, Vredeveld EJ, Credoe HM, et al. Whole breast proton irradiation for maximal reduction of heart dose in breast cancer patients. Breast Cancer Res Treat 2014;148:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hardee ME, Raza S, Becker SJ, et al. Prone hypofractionated whole-breast radiotherapy without a boost to the tumor bed: comparable toxicity of IMRT versus a 3D conformal technique. Int J Radiat Oncol Biol Phys 2012;82:e415–23. [DOI] [PubMed] [Google Scholar]
- 145.Shaitelman SF, Howell RM, Smith BD. Effects of Smoking on Late Toxicity From Breast Radiation. J Clin Oncol 2017;35:1633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin LL, Vennarini S, Dimofte A, et al. Proton beam versus photon beam dose to the heart and left anterior descending artery for left-sided breast cancer. Acta Oncol 2015;54:1032–9. [DOI] [PubMed] [Google Scholar]
- 147.Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol 2002;62:137–45. [DOI] [PubMed] [Google Scholar]
- 148.Rodgers B, Jaroszewski D, Ashman J, Rule W, Sio T, Keole S. Advantages of postmastectomy proton beam therapy in a breast cancer patient with pectus excavatum. Journal of Medical Cases 2017;8:98–101. [Google Scholar]
- 149.Thorpe CS, Niska JR, Anderson JD, et al. Acute toxicities after proton beam therapy following breast-conserving surgery for breast cancer: Multi-institutional prospective PCG registry analysis. Breast J 2020. [DOI] [PubMed] [Google Scholar]
- 150.Jobsen JJ, van der Palen J, Ong F, Riemersma S, Struikmans H. Bilateral breast cancer, synchronous and metachronous; differences and outcome. Breast Cancer Res Treat 2015;153:277–83. [DOI] [PubMed] [Google Scholar]
- 151.Heron DE, Komarnicky LT, Hyslop T, Schwartz GF, Mansfield CM. Bilateral breast carcinoma: risk factors and outcomes for patients with synchronous and metachronous disease. Cancer 2000;88:2739–50. [PubMed] [Google Scholar]
- 152.Kaidar-Person O, Kostich M, Zagar TM, et al. Helical tomotherapy for bilateral breast cancer: Clinical experience. Breast 2016;28:79–83. [DOI] [PubMed] [Google Scholar]
- 153.Vyfhuis MAL, Zhu M, Agyepong B, Nichols EM. Techniques for Treating Bilateral Breast Cancer Patients Using Pencil Beam Scanning Technology. Int J Part Ther 2019;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun T, Lin X, Tong Y, et al. Heart and Cardiac Substructure Dose Sparing in Synchronous Bilateral Breast Radiotherapy: A Dosimetric Study of Proton and Photon Radiation Therapy. Front Oncol 2019;9:1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Depauw N, Batin E, Johnson A, MacDonald S, Jimenez R. Arms positioning in postmastectomy proton radiation: feasibility and development of a new arms down contouring atlas. Physics and Imaging in Radiation Oncology 2020;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Belkacemi Y, Hanna NE, Besnard C, Majdoul S, Gligorov J. Local and Regional Breast Cancer Recurrences: Salvage Therapy Options in the New Era of Molecular Subtypes. Front Oncol 2018;8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kaidar-Person O, Oldenborg S, Poortmans P. Re-irradiation and Hyperthermia in Breast Cancer. Clin Oncol (R Coll Radiol) 2018;30:73–84. [DOI] [PubMed] [Google Scholar]
- 158.Thangarajah F, Heilmann J, Malter W, et al. Breast conserving surgery in combination with intraoperative radiotherapy after previous external beam therapy: an option to avoid mastectomy. Breast Cancer Res Treat 2018;168:739–44. [DOI] [PubMed] [Google Scholar]
- 159.Oldenborg S, Rasch CRN, van Os R, et al. Reirradiation + hyperthermia for recurrent breast cancer en cuirasse. Strahlenther Onkol 2018;194:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chin C, Jadeja P, Taback B, et al. Evaluation of Partial Breast Reirradiation with Intraoperative Radiotherapy after Prior Thoracic Radiation: A Single-Institution Report of Outcomes and Toxicity. Front Oncol 2017;7:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Arthur DW, Winter KA, Kuerer HM, et al. NRG Oncology-Radiation Therapy Oncology Group Study 1014: 1-Year Toxicity Report From a Phase 2 Study of Repeat Breast-Preserving Surgery and 3-Dimensional Conformal Partial-Breast Reirradiation for In-Breast Recurrence. Int J Radiat Oncol Biol Phys 2017;98:1028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Harkenrider MM, Wilson MR, Dragun AE. Reirradiation as a component of the multidisciplinary management of locally recurrent breast cancer. Clin Breast Cancer 2011;11:171–6. [DOI] [PubMed] [Google Scholar]
- 163.Plastaras JP, Berman AT, Freedman GM. Special cases for proton beam radiotherapy: reirradiation, lymphoma, and breast cancer. Semin Oncol 2014;41:807–19. [DOI] [PubMed] [Google Scholar]
- 164.. Thorpe CS, Niska JR, Girardo ME, et al. Proton beam therapy reirradiation for breast cancer: Multi-institutional prospective PCG registry analysis. Breast J 2019;25:1160–70. [DOI] [PubMed] [Google Scholar]
- 165.Fernandes A, Berman AT, Mick R, et al. A Prospective Study of Proton Beam Reirradiation for Esophageal Cancer. Int J Radiat Oncol Biol Phys 2016;95:483–7. [DOI] [PubMed] [Google Scholar]
- 166.Badiyan SN, Rutenberg MS, Hoppe BS, et al. Clinical Outcomes of Patients With Recurrent Lung Cancer Reirradiated With Proton Therapy on the Proton Collaborative Group and University of Florida Proton Therapy Institute Prospective Registry Studies. Pract Radiat Oncol 2019;9:280–8. [DOI] [PubMed] [Google Scholar]
- 167.Chao HH, Berman AT, Simone CB 2nd, et al. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:281–92. [DOI] [PubMed] [Google Scholar]
- 168.Fattahi S, Ahmed SK, Park SS, et al. Reirradiation for Locoregional Recurrent Breast Cancer. Adv Radiat Oncol 2021;6:100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wahl AO, Rademaker A, Kiel KD, et al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys 2008;70:477–84. [DOI] [PubMed] [Google Scholar]
- 170.Mailhot Vega RB, Ishaq O, Raldow A, et al. Establishing Cost-Effective Allocation of Proton Therapy for Breast Irradiation. Int J Radiat Oncol Biol Phys 2016;95:11–8. [DOI] [PubMed] [Google Scholar]
- 171.Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer 2016;122:1483–501. [DOI] [PubMed] [Google Scholar]
- 172.Lundkvist J, Ekman M, Ericsson SR, Jonsson B, Glimelius B. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol 2005;44:850–61. [DOI] [PubMed] [Google Scholar]
- 173.Mailhot Vega RB, Wu SP, Ishaq O, et al. ost-Effectiveness and Value of Information Analyses of Proton versus Photon Comprehensive Radiotherapy for Stage I-III Breast Cancer. American Society for Radiation Oncology National Meeting 2017. San Diego, CA2017. [Google Scholar]
- 174.Ovalle V, Strom EA, Godby J, et al. Proton Partial-Breast Irradiation for Early-Stage Cancer: Is It Really So Costly? Int J Radiat Oncol Biol Phys 2016;95:49–51. [DOI] [PubMed] [Google Scholar]
- 175.Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020;395:1613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086–94. [DOI] [PubMed] [Google Scholar]
- 177.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513–20. [DOI] [PubMed] [Google Scholar]
- 178.Bekelman JE, Hahn SM. Reference pricing with evidence development: a way forward for proton therapy. J Clin Oncol 2014;32:1540–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou Q, Howard ME, Tu X, et al. Inhibition of ATM induces hypersensitivity to proton irradiation by upregulating toxic end joining. Cancer Res 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]