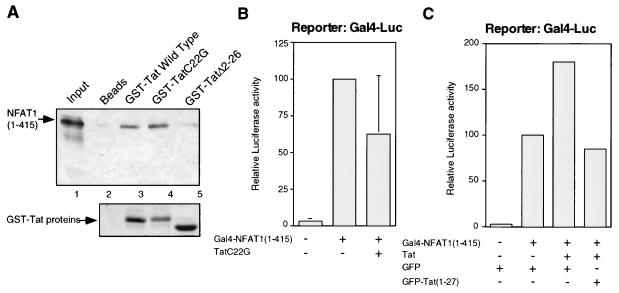
FIG. 3.
HIV-1 Tat enhancement of NFAT1 transactivation requires a transcriptionally active Tat protein and is mediated by the first 26 amino acids of Tat. (A) The binding affinities of two mutated Tat proteins (TatC22G and TatΔ2–26) for the amino-terminal domain of NFAT1 were assayed. Binding reaction mixtures were resolved on an SDS-acrylamide gel and blotted. The bound products were detected with an antibody against an amino-terminal peptide of NFAT1 (67.1). A Ponceau red staining of the blot showing the amounts of Tat proteins used in the binding reaction mixtures is shown below the immunoblot. (B) An inactive Tat protein does not stimulate NFAT1-mediated transactivation. Jurkat cells were transfected with a GAL4-Luc reporter plasmid (2 μg) and pGAL4-NFAT1(1–415) (2.5 μg), with or without a plasmid expressing a mutant (Cys22-to-Gly) Tat protein (0.5 μg). Cells were stimulated for 8 h with 10 nM PMA and 2 μM ionomycin. Total amounts of DNA were adjusted with the appropriate empty vector. Values are means + standard errors of results from three independent experiments. (C) Expression of a GFP-Tat(1–27) fusion protein inhibits Tat-mediated upregulation of NFAT1 transactivation. Jurkat cells were transfected with a GAL4-Luc reporter plasmid (2 μg), pGAL4-NFAT1(1–415) (2.5 μg), pcTat (0.5 μg), and pEGFP or pEGFPTat(1–27) (2 μg). Cells were stimulated for 8 h with 10 nM PMA and 2 μM ionomycin. Values are the means of results from two independent experiments.