Figure 1.
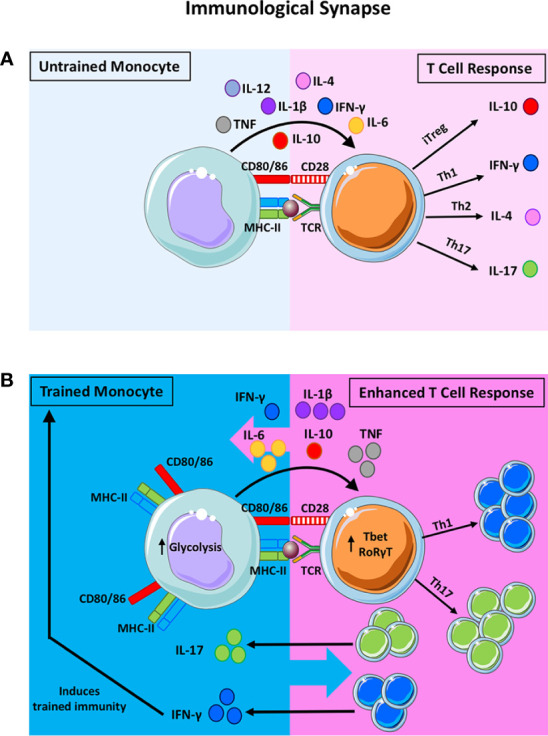
The potential impact of trained immunity on the bidirectional innate-adaptive immune synapse. The traditional synapse between antigen-presenting cells (APC) and naïve T helper cells is depicted in (A). The innate APC processes and presents antigen on the MHC-II molecule to provide the first signal to activate the T cell. Upregulation of co-stimulatory molecules on the APC and their ligation to the cognate receptors on the T cells provide the second signal required for T cell activation. Cytokine production by the innate APC then polarises the naïve T cell into discreet subsets. For example, the presence of IL-10 will induce the formation of T regulatory cells (Treg) whereas IL-12 and IFN-γ will polarise T cells towards a Th1 lineage. IL-4 promotes Th2 polarisation and IL-1β and IL-6 (along with other innate cytokines) promote Th17 development. This synapse is often viewed as unidirectional, however, there is evidence to suggest that T cell cytokines can impact also APC function. In addition to being the critical control point in the polarization of naïve T cells, this synapse is also crucial for reactivating memory T cells in tissues. Under these circumstances, innate cytokine production can alter the functional profile of previously lineage committed T cells. When trained immunity is induced in innate cells, their APC function and cytokine production is markedly enhanced (B). Trained innate cells exhibit increased expression of MHC-II, costimulatory molecules (CD80/CD86) and an upregulation of aerobic glycolysis. Moreover, trained monocytes produce significantly more TNF, IL-1β and IL-6 compared with untrained monocytes. These features of proinflammatory innate immune training suggest that T cell polarization may be skewed in favour of Th17/Th1 responses, as indicated by the expression of the master transcription factors RORγT and Tbet, respectively. In the same manner, lineage committed T cells that are prone to functional plasticity, such as the Th17 and Treg lineages, may be altered by trained immunity and directed down alternative lineage fates. Enhanced IFN-γ and IL-17 production from T cells can impact innate cell function. In fact, IFN-γ production has been shown to induce trained immunity in tissue resident macrophages. Notably, alternative types of trained immunity that result in enhanced IL-10, for example, will alter T cell fate towards Treg cells, which may in turn impact innate APC function. We propose that, trained immunity alters T cell fate and function which may in turn alter innate cell function. Depending on the primary insult that induces trained immunity, the pro-inflammatory versus anti-inflammatory nature of this bi-directional synapse will be altered depending on the cytokine milieu and the cellular functions that are induced by trained immunity.
