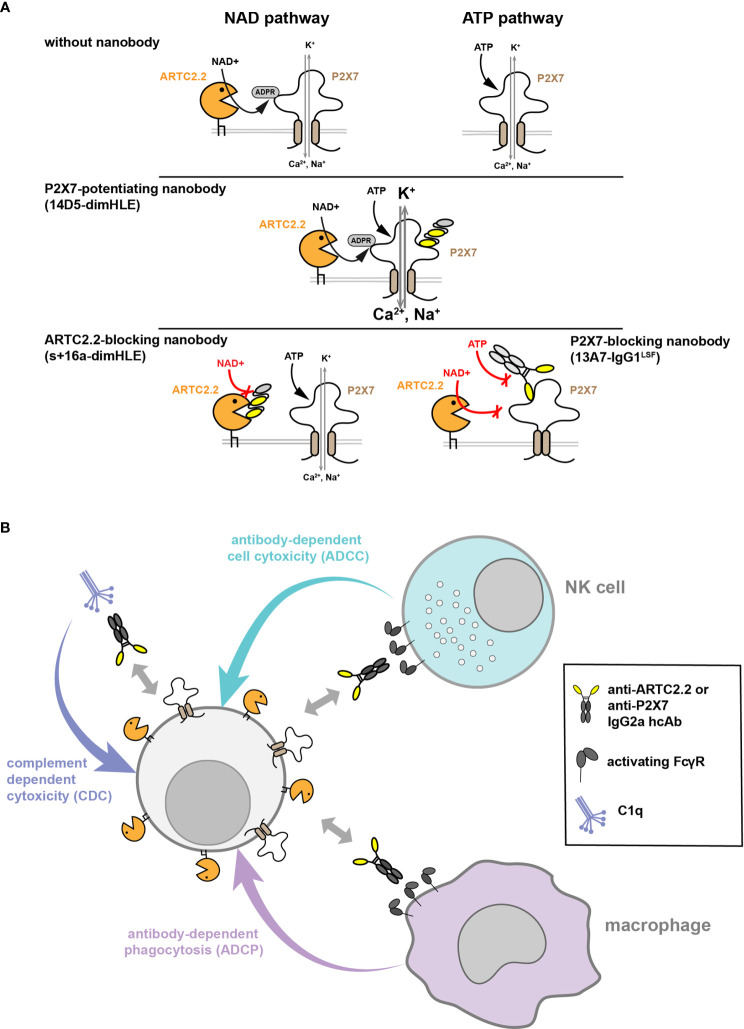
Figure 2.
Strategies used to manipulate ARTC2.2 or P2X7 functions in vivo or to deplete T cells expressing these proteins. (A) Modulation of ARTC2.2 and P2X7 activity using nanobody-based constructs. Two pathways can lead to the activation of P2X7 at the surface of mouse T cells. The ATP pathway involves direct gating of P2X7 ion channel by extracellular ATP. The NAD+ pathway involves a post-translational modification of P2X7 catalyzed by the ectoenzyme ARTC2.2 resulting in its covalent ADP-ribosylation. The 14D5-dimHLE construct probably binds to an allosteric site on P2X7 and potentiates gating of the ion channel triggered by either ATP or NAD+. The s+16a-dimHLE construct was designed to block ARTC2.2 enzymatic activity in vivo and thereby to inhibit activation of P2X7 by the NAD+ pathway (but not the ATP pathway). The 13A7-IgG1LSF hcAb construct inhibits P2X7 gating and can be used to inhibit both ATP and NAD+ pathways. (B) Other nanobody-based constructs were designed to favor depletion of their target cells in vivo. For that, nanobodies s-14 and 7E2, that bind respectively to ARTC2.2 and P2X7 but do not modulate their functions, were fused to the hinge and Fc-region of mIgG2a. The resulting mIgG2a hcAb could mediate cell depletion through different mechanisms such as complement-dependent cytotoxicity (CDC), antibody-dependent cell cytotoxicity (ADCC) that rely on NK cells, and antibody-dependent cell phagocytosis (ADCP) involving macrophages.