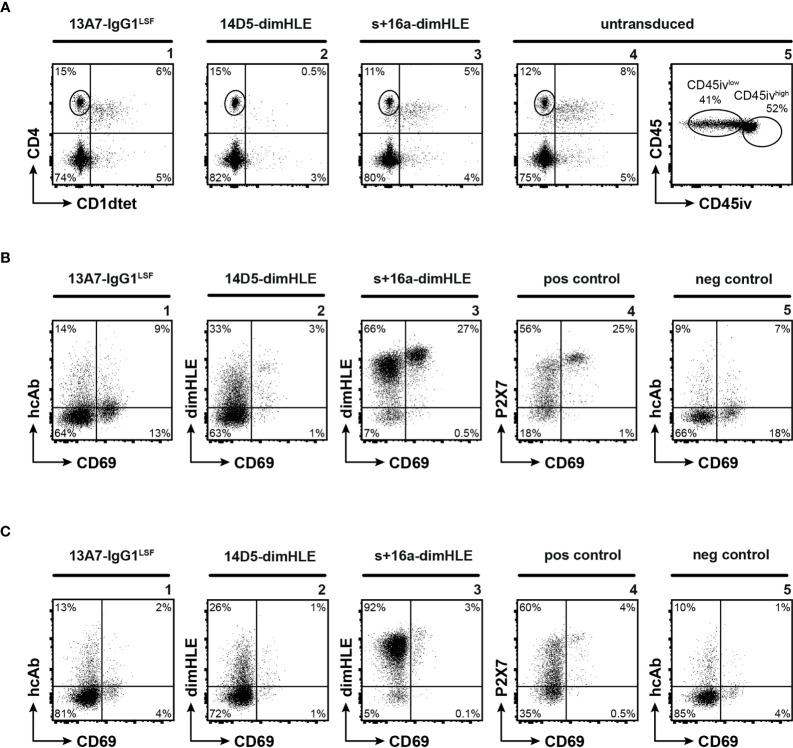
Figure 4.
Nanobody-based biologics produced in vivo following rAAV injection reach ARTC2.2 and P2X7 expressed at high levels by TRM located in the liver parenchyma. C57BL/6 mice were injected i.m. with rAAV1 encoding P2X7-blocking 13A7-IgG1LSF hcAb, P2X7-potentiating 14D5-dimHLE, or ARTC2.2-blocking s+16a-dimHLE, or with PBS (untransduced). 120 days after rAAV injection mice received an intravenous injection of fluorochrome-conjugated CD45-specific mAb (CD45iv) 3 min before sacrifice. Untransduced mice additionally received 50 µg of recombinant s+16a-dim construct 30 min before organ collection to prevent NICD and cell loss ex vivo during cell preparation, as described earlier (15, 18). Cells extracted from the liver were then counterstained with mAbs directed against CD45, CD4, and CD69 (i.e., a marker of tissue resident lymphocytes) and with a CD1d-tetramer (CD1dtet) loaded with αGal/Cer (specifically labels the invariant T cell receptor of NKT cells). (A) Representative flow cytometry plots illustrating liver CD4+ T cells and CD4+CD1dtet+ NKT cells (panels 1-4), and the gating of parenchymal (CD45ivlow) and vascular (CD45ivhigh) CD4+CD1dtet- lymphocytes (panel 5). (B, C) Representative flow cytometry plots illustrating the detection of the nanobody-based constructs on the cell surface of tissue resident CD45ivlow (B) and vascular CD45ivhigh (C) CD4+CD1dtet- T cells. To detect cell surface bound dimHLE constructs, cells were stained with Alb8-specific mAb77 (mouse IgG1) followed by an mIgG1-specific secondary mAb. To detect cell surface bound 13A7-IgG1LSF, cells were stained directly with the secondary mIgG1-specific mAb. Positive (pos) control staining was performed with liver cells from untransduced mice using the P2X7-specific 13A7-dimHLE, mAb77, and the mIgG1-specific secondary mAb. Negative (neg) control staining was performed with the secondary detection reagents alone. Numbers indicate the percentages of cells in the respective quadrants or gates (A, panel 5). Staining was performed using fluorochrome conjugated antibodies specific to CD45 (coupled to PerCP-Cy5.5 for the one injected i.v. and to AF700 for the one added in vitro), CD4 (APC), CD69 (FITC), mouse IgG1 (BV421) and CD1dtet (coupled to PE).