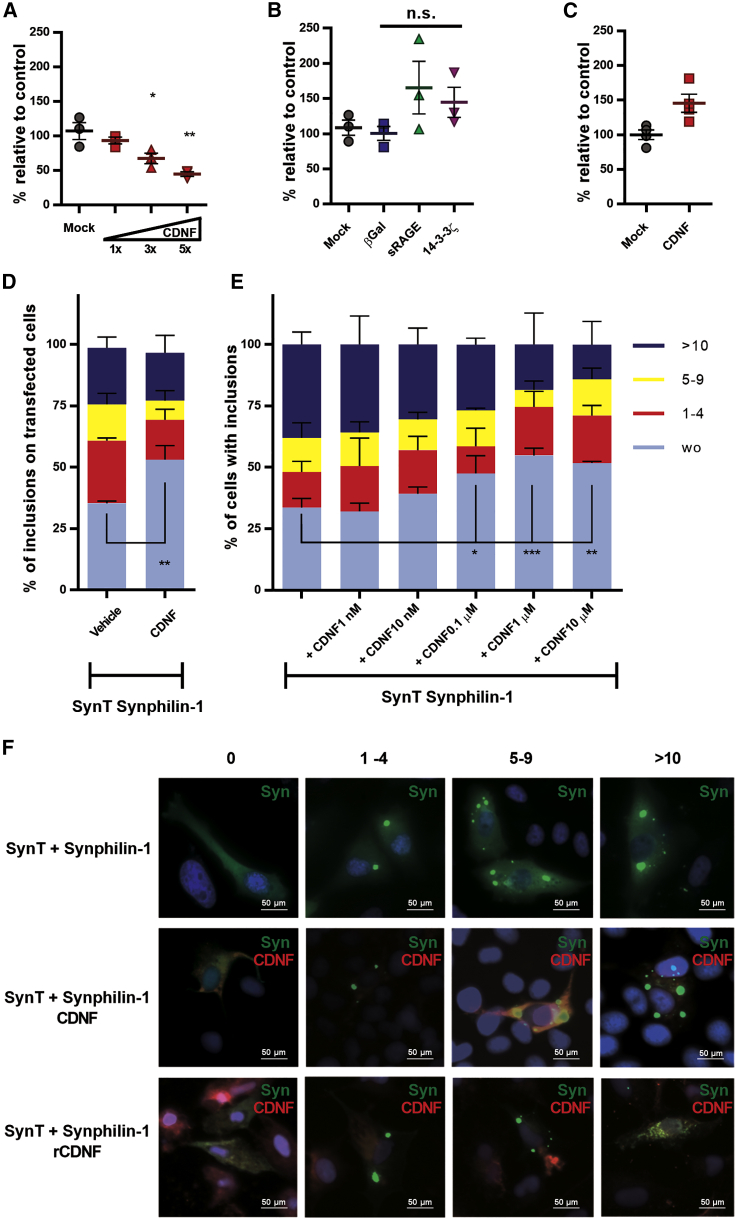
Figure 1.
Effect of CDNF on α-synuclein auto association in cells
(A–C) Neuro2A cells were transiently transfected with a pair of reporter plasmids expressing human wild-type α-synuclein fused to one of the split Gaussia luciferase (GLuc) fragments. Upon oligomerization, the interaction allows reconstitution of luciferase activity from the complementary GLuc fragments. (A) Co-expression of CDNF with its original signal sequence (mostly ER luminal protein) dose-dependently reduced α-synuclein oligomerization. Doses of CDNF expression plasmid 1× = 12.5 ng, 3× = 37.5 ng, 5× = 62.5 ng. (B) Co-expression of β-galactosidase (mainly lysosomal protein), soluble receptor for advanced glycation end products (sRAGE; a secreted protein), and 14-3-3ζ (a cytosolic protein) did not significantly alter α-synuclein oligomerization. (C) Cells co-transfected with full-length (GLuc) enzyme together with CDNF did not show decreased luciferase activity as compared to cells co-transfected with a mock plasmid. (D–F) Cells co-transfected with SynT/Synphilin-1 and CDNF with its original signal sequence, grown for 48 h (D and lines 1 and 2 in F) or pre-treated with recombinant human CDNF (E and line 3 in F) before the transient transfections with SynT/Synphilin-1. Cells were processed for immunocytochemistry with antibodies against human α-synuclein (green) or CDNF (red) and analyzed by fluorescence microscopy for the presence of inclusions. Cells were categorized according to the number of inclusions per cell: cells without inclusions (wo), between 1 to 4 (1–4), 5 to 9 (5–9), or more than 10 inclusions (>10). The scale bars represent 50 μm. The ordinary one-way ANOVA test was performed to analyze the differences between the various groups. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001. Error bars represent SEM.