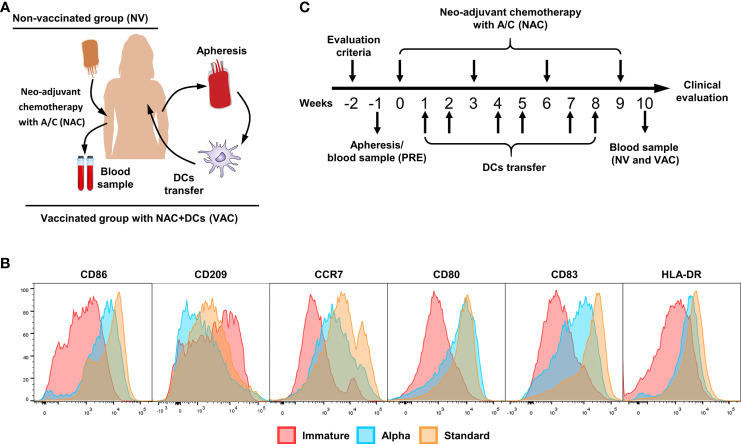
Figure 1.
Vaccination scheme and dendritic cell (DC) characterization. (A) Patient interventions, before NAC, patients have gone to an apheresis collection to obtain peripheral blood mononuclear cells (PBMCs) and therefore induce monocyte-derived DCs (cryopreserved until use). After four doses of chemotherapy, a new blood sample was taken to compare between pre- and post-chemotherapy. (B) Representative histograms of immature DCs (red), alpha DCs (blue), and standard DCs (yellow), comparing the expression levels of CD86, CD209, CCR7, CD80, CD83, and HLA-DR. (C) Clinical trial scheme per patient in weeks, week 0 is referred to the first dose of chemotherapy, the process starts with the evaluation criteria for patients (w-2), apheresis (vaccinated group) or blood sample (control group) was taken in w-1. Between doses of chemotherapy, we transferred two doses of DCs for a total of six doses. One week after the fourth dose of A/C, we collect the second blood sample.