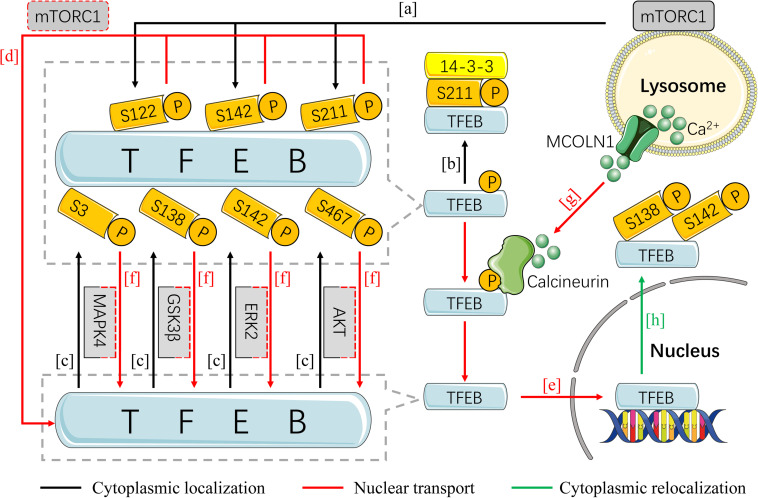
FIGURE 1.
The regulation of TFEB activity by phosphorylation. In the condition of nutritional sufficiency, TFEB is isolated outside the nucleus in an inactive state by phosphorylation and accumulates in the cytoplasm. TFEB can be phosphorylated by mTORC1 on S122, S142, and S211 (A), by AKT on S467, and by GSK3β on S138, by ERK2 on S142, and by MAPK4 on S3 (C). The phosphorylated TFEB on S211 is recognized and bound by YWHA/14-3-3 protein in atypical patterns (B). When encountering with starvation or stress, the withdraw of any phosphorylation site is sufficient to induce TFEB activation and nuclear transport (D,F). Calcineurin is stimulated by the Ca2+ released from the lysosome in MCOLN1-dependent way and is primarily responsible for the dephosphorylation of TFEB (G). Dephosphorylated TFEB transfers to nucleus and further initiates the transcription of target genes (E). Refeeding after nutrition deprivation, TFEB initiates cytoplasmic relocalization in a short time by hierarchical phosphorylation on S142 and S138 (H). (AKT, protein kinase B; ERK2, extracellular regulated protein kinase 2; GSK3β, glycogen synthase kinase 3β; MAPK4, mitogen-activated protein kinase 4; mTORC1, mammalian target of rapamycin complex 1; TFEB, transcription factor EB).