Figure 2.
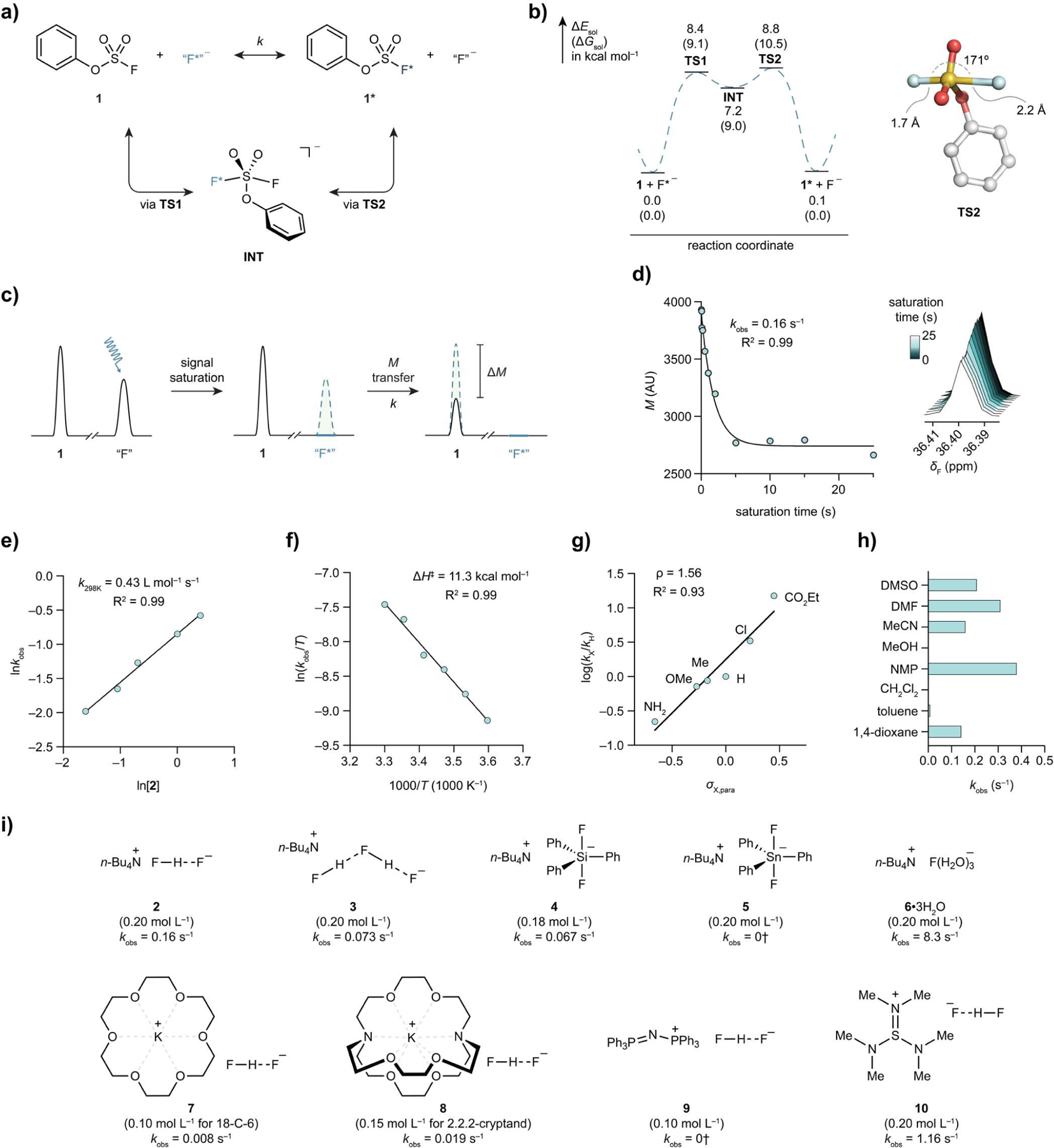
Investigation of sulfur fluoride exchange between phenyl fluorosulfate and a fluoride salt. (a) SuFEx of phenyl fluorosulfate (1). “F−” denotes any nucleophilic fluoride donor, exemplified by those shown below (i). (b), DFT calculated energy profile of a typical SuFEx process in acetonitrile and the optimized geometry of the highest transition state TS2. (c), Schematic illustration of TDST-NMR. (d), A representative plot of magnetization versus saturation time, and overlapped spectra (right) of the corresponding experiments with saturation time ranging from 0.01 s to 25 s. Conditions: phenyl fluorosulfate (1, 0.02 mol L−1), tetrabutylammonium bifluoride (2, 0.2 mol L−1), MeCN-d3, 298 K. kobs = 0.16 s−1 (R2 = 0.99). (e), Measurement of the second-order rate of the SuFEx reaction between 1 and 2, k298K = 0.43 L mol−1 s−1 (R2 = 0.99). (f) Erying plot of the SuFEx reaction between 1 and 2. The calculated activation enthalpy was determined, ΔH‡calc = 11.3 kcal mol−1 (R2 = 0.99). (g) Hammett plot of the SuFEx reactions between 2 and para-X-substituted phenyl fluorosulfates, ρ = 1.56 (R2 = 0.93). (h) Solvents effect. (i) Structure-activity relationship of various fluoride salts (2–10) on the SuFEx reaction of 1. †No exchange detected by NMR.
