Figure 3.
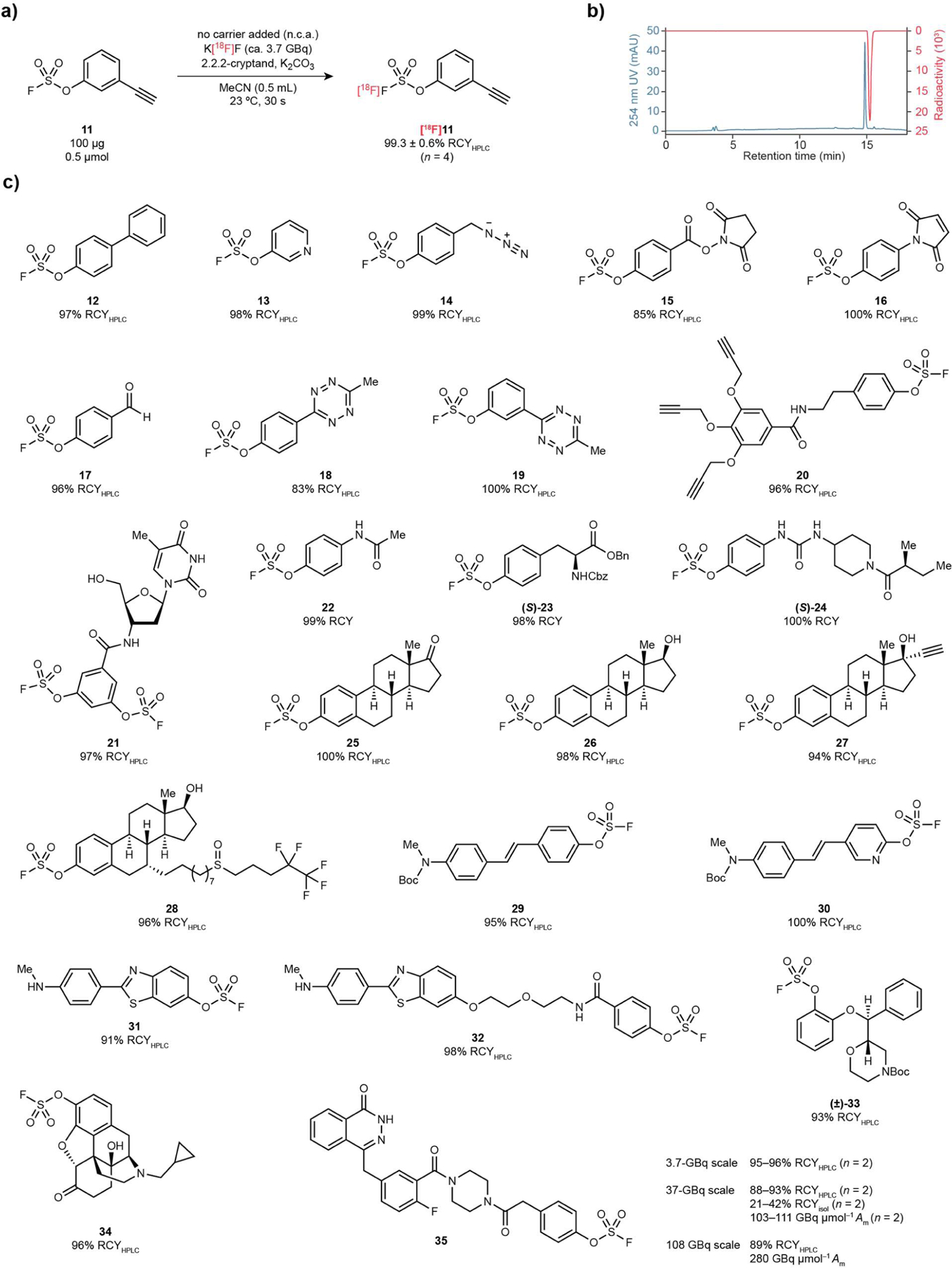
[18F]SuFEx of aryl fluorosulfates. (a) [18F]SuFEx of 3-ethynylphenyl fluorosulfate (11). Conditions: Compound 11 (0.1 mg), K[18F]F (ca. 3.7 GBq), [2.2.2]-cryptand, K2CO3, MeCN (0.5 mL), 23 °C, 30 s. RCYHPLCs were determined by HPLC (n = 4) after the reaction crude been quenched by water (0.1 mL). (b) Representative HPLC chromatograms of a reaction crude of [18F]11, with 254 nm UV absorption (blue) and radioactivity (red) traces. (c) Substrate scope of the [18F]SuFEx reaction following the conditions described before in (a). Curie-scale synthesis of [18F]35. Conditions: Compound 35 (0.1 mg, 17.2 nmol), K[18F]F (~37 GBq or 111 GBq), [2.2.2]-cryptand, K2CO3, MeCN (10 mL), 23 °C, 30 s.
