Abstract
Background
Glucocorticoids modulate the surgical stress response. Previous studies showed that high-dose preoperative glucocorticoids reduce levels of postoperative inflammatory markers and specific biomarkers of liver damage compared with placebo, and suggested a reduced complication rate and shorter hospital stay after liver surgery. However, there are no studies with a clinical primary outcome or of early recovery outcomes. The aim of this study was to investigate whether a single high dose of preoperative glucocorticoid reduces complications in the immediate postoperative phase after liver surgery.
Methods
This was a single-centre, double-blinded, parallel-group RCT investigating preoperative methylprednisolone 10 mg/kg (high dose) versus dexamethasone 8 mg (standard-dose postoperative nausea prophylaxis) in patients scheduled for open liver resection. The primary outcome was number of patients with a complication in the postanaesthesia care unit; secondary outcomes included duration of hospital stay, pain and nausea during admission, and 30-day morbidity.
Results
A total of 174 patients (88 in high-dose group, 86 in standard-dose group) were randomized and analysed (mean(s.d.) age 65(12) years, 67.2 per cent men); 31.6 per cent had no serious co-morbidities and 25.3 per cent underwent major liver resection. Complications occurred in the postanaesthesia care unit in 51 patients (58 per cent) in the high-dose group and 58 (67 per cent) in the standard-dose group (risk ratio 0.86, 95 per cent c.i. 0.68 to 1.08; P = 0.213). Median duration of hospital stay was 4 days in both groups (P = 0.160). Thirty-day morbidity and mortality rates were similar in the two groups.
Conclusion
A high dose of preoperative glucocorticoids did not reduce acute postoperative complications after open liver resection compared with a standard dose. Registration number: NCT03403517 (http://www.clinicaltrials.gov); EudraCT 2017–002652-81 (https://eudract.ema.europa.eu/).
High-dose preoperative glucocorticoids, which have been shown to alleviate the surgical stress response, do not seem to be superior to standard low-dose glucocorticoids in preventing acute complications after open liver surgery
Introduction
Liver resection is performed mainly for primary (hepatocellular carcinoma and cholangiocellular carcinoma) or secondary (colorectal cancer metastases) tumours. Advances in surgical techniques and perioperative optimization in fast-track protocols have reduced morbidity and mortality, with a subsequent reduction in duration of hospital stay1. However, the existing preoperative morbidity of most patients undergoing liver resection and the extent of the surgical procedure means that recovery still represents a challenge, with postoperative complication rates in up to 40 per cent depending on definitions and time frame2–6. Furthermore, postoperative complications are related to the extent of surgery, type of anaesthesia, any intraoperative complications, and potentially also to the extent of the surgical stress response in the immediate postoperative phase7,8. The surgical stress response can theoretically be reduced with use of preoperative glucocorticoids (GCs), by the downregulation of proinflammatory proteins and upregulation of anti-inflammatory proteins, resulting in an overall negative feedback on immunostimulation9,10. Several studies6,11–13 have reported the effect of preoperative GCs compared with controls in reducing inflammatory cytokines and biomarkers of liver damage after liver resection. The GC effect on these surrogate outcomes has been shown to be statistically significant mostly on postoperative day (POD) 1, but is also evident immediately after surgery when measured within the first postoperative hours12,13. There is no clear evidence as to whether inflammatory control is related to a reduction in early clinically relevant complications, although a recent study14 of preoperative GC administration before liver resection reported a significantly lower 90-day complication rate in the GC compared with a placebo group. Similarly, previous studies6,11,14 have reported a lower overall morbidity rate6 and a shorter hospital stay in the GC intervention groups, suggesting that there might be an effect on these clinical outcomes in liver resectional surgery as well as in other surgical procedures15–20. No previous studies have described the effects in the immediate postoperative phase.
To investigate the preventive effects of GCs on adverse clinical outcomes, the focus of the present study was on the immediate postoperative phase during the postanaesthesia care unit (PACU) stay. Here, all patients are monitored closely, treated, and discharged according to well defined protocols, allowing straightforward comparisons between groups and evaluation of the effects on early recovery. The aim of this study, therefore, was to investigate whether a high dose of GC (10 mg/kg methylprednisolone), given as a single preoperative injection, could reduce complications in the immediate phase after liver resection compared with a standard low-dose of GC used for postoperative nausea and vomiting (PONV) prophylaxis (8 mg dexamethasone). The primary outcome was the number of patients with complications requiring treatment during observation in the PACU according to predefined criteria. An additional hypothesis was that high-dose GCs would reduce the duration of stay in the PACU, and reduce pain scores and opioid consumption in the first 3–4 days after surgery without increasing 30-day morbidity.
Methods
This single-centre, double-blinded, parallel-group RCT was approved by the local ethics committee (H-17025897), the Danish data protection agency (RH-2017–108), and the Danish Medicines Agency before patient enrolment. The trial was registered at ClinicalTrials.gov (NCT03403517) and EudraCT (2017–002652-81), and was monitored by an independent good clinical practice unit. The trial was conducted at Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark, between December 2017 and September 2020. The English version of the study protocol can be found in Appendix S1.
Eligibility criteria
All patients scheduled for open liver surgery without biliary reconstruction were consecutively screened for inclusion. Patients considered were 18 years of age or older, and able to provide informed oral and written consent. Exclusion criteria were: planned simultaneous operation on other organs, simultaneous operation for hernia with insertion of mesh, planned two-stage or associating liver partition and portal vein ligation for staged hepatectomy, active hepatitis C virus infection, daily/current use of GCs or immunosuppressant medication (within 10 days before the procedure), insulin-dependent diabetes, and pregnancy or lactation.
Randomization and blinding
Patients were informed by trial personnel in relation to the preoperative appointment, usually 1–2 weeks before the operation, and included in the trial on the day of surgery after providing written consent. Participants were stratified according to the planned extent of surgery (major resections of at least 3 liver segments, minor resections of fewer than 3 liver segments) and randomized 1 : 1 in parallel groups by block randomization. Block sizes (4, 6, 8) varied and were unknown to study personnel. The allocation sequence was generated by an independent physician using Sealed Envelope (https://www.sealedenvelope.com/simple-randomiser/v1/lists). The allocation sequence with intervention details was concealed in consecutively numbered opaque envelopes by two other investigators not otherwise involved in the trial. Before sealing, 20 per cent of the envelopes were randomly controlled, and the allocation sequence was stored by a physician not otherwise involved in the trial. After trial completion, the principal investigator received the allocation sequence without the intervention being revealed. The intervention allocation was revealed after undertaking statistical analysis and drafting the paper, including results, conclusion, and comments from all authors. Throughout the trial, all participants, trial personnel, healthcare providers including surgeons, anaesthetists and nurses, outcome assessors, and data monitor were blinded. There were no code breaks during the trial.
Intervention
The high-dose (HD) group received 10 mg/kg methylprednisolone (Solu-medrol®, Pfizer, Ballerup, Denmark) and the standard-dose (SD) group received 8 mg dexamethasone (Dexavit; Vital Pharma Nordic, Denmark). On the day of surgery, personnel assigned only to prepare the study drugs received and opened the sealed envelope, and prepared the trial drug. The study medication in both groups was injected in a sterile (drip) bag containing 100 ml sodium chloride, covered in foil to conceal the content and amount. The bag was labelled with a patient identifier and handed to trial personnel together with the resealed and signed envelopes. Trial personnel administered the medication over 10 min immediately after induction of anaesthesia, around 30 min before surgery. The drip was flushed with sodium chloride at the end of administration of the trial drug.
Surgery, anaesthesia, and standard regimens
All patients were treated according to a well implemented enhanced recovery after surgery (ERAS) protocol, as described previously1,21. Before surgery, all patients had an epidural analgesia catheter inserted at thoracic level 8–10, which was maintained with a continuous infusion of bupivacaine/morphine (2.5 mg + 50.0 µg/ml). After induction, a 30-mg bolus of 0.5 per cent bupivacaine was administered, followed by 15 mg 0.5 per cent bupivacaine every hour during surgery. Anaesthesia was induced with propofol (1.5–2.5 mg/kg), remifentanil (0.3–0.8 µg/kg), and cisatracurium (0.1 mg/kg) or suxamethonium (1 mg/kg). Anaesthesia was maintained with propofol 0.05–0.15 mg per kg per min, remifentanil (0.3–0.8 μg per kg per min) and cisatracurium, aiming at a train-of-four (TOF) response of 0 per cent. In patients with impaired liver function in combination with major resection, sevoflurane could replace propofol. A noradrenaline (norepinephrine) infusion was administered to maintain mean arterial pressure (MAP) above 60 mmHg. During resection, central venous pressure (CVP) was maintained at around 4–5 mmHg. Ondansetron (4 mg) was administered 30–45 min before the end of surgery. Before extubation, a TOF ratio of more than 90 per cent was confirmed.
Monitoring included invasive arterial BP, CVP, ECG, pulse oximetry, nasopharyngeal temperature, nerve stimulator, urinary catheter, and a nasogastric tube.
The surgical procedure was preferably performed through a curved subcostal incision extended in the midline to the xiphoid process (thoracic 7–10). For resections in the left lateral segment, an upper midline incision was used. If hepatic clamping was performed, an intermittent Pringle manoeuvre was applied for a maximum of 15 min each time, with 5-min intervals in between if repeated. Perioperative antibiotic comprised a single dose of 2 g ceftriaxone. In the event of blood loss exceeding 1000 ml, it was continued as 1500 mg cefuroxime three times daily and metronidazole 1500 mg once daily for 3 days. Drains were placed only if there was intraoperative bleeding or an assumed risk of bile leakage, with removal planned for POD 1 if possible. Low molecular weight heparin was initiated on the morning of surgery and continued until full mobilization. Nasogastric tubes were removed immediately after the procedure in the operating room. The radial artery cannula was removed on POD 0 before discharge from the PACU; the central venous catheter was removed on POD 1 after minor resections, and POD 3 after major resections. The urinary catheter was removed on POD 1.
Postoperative pain management consisted of epidural infusion of a bupivacaine/morphine (2.5 mg + 50.0 µg/ml) infusion starting during surgery at 5 ml/h but titrated depending on effect/adverse effects and continued for a maximum of 3 postoperative days. Modified-release acetaminophen 1330 mg every 8 h was started immediately after surgery (individual prescription in the major resection group). On the evening of POD 2, additional 200 mg celecoxib and gabapentin 600 mg was started (reduced dose if weight below 50 kg or age over 65 years or in patients with impaired renal function), and continued every 12 h (celecoxib 200 mg, gabapentin 300 mg in the morning and 600 mg in the evening) for 1 week.
If the postoperative course was uneventful, discharge was expected and planned on POD 3 or 4.
Primary outcome
The primary endpoint was the number of patients with a complication requiring treatment in the PACU, defined according to a standardized postanaesthesia discharge score, a modified version of the Aldrete discharge score22 (Table S1). The score comprises six modalities: sedation, oxygen saturation, BP, heart rate, pain (at rest), and nausea.
A PACU complication was defined by any score exceeding 1 (except more than 2 for oxygen saturation), on two consecutive measurements 30 min apart or a score greater than 1 at a single time point accompanied by a relevant treatment (such as pain score on a numerical rating scale (NRS) over 3, accompanied by a rescue opioid administration, bradycardia or tachycardia accompanied by frequency regulation/conversion for arrythmia, PONV accompanied by antiemetic treatment) or relevant treatment for more than 30 min regardless of score (continuous inotropic infusion for hypotension, oxygen supply exceeding 2 litres or other respiratory treatment).
Secondary outcomes
Secondary outcomes were: all cause 30-day mortality; 30-day morbidity (liver failure, ascites, intra-abdominal fluid collection, bleeding, cholascos, bowel obstruction, perforated visceral organ, fascial disruption, other reasons for reoperation, pleural effusion, pulmonary embolus, deep venous thrombosis, acute myocardial infarction, transitory cerebral ischaemia, stroke, infections (pneumonia, urinary tract infection, sepsis, wound infection), other reasons for prolonged hospital stay); number of patients with a complication requiring treatment (procedural, medication, alterations in standard care) during the first 24 h after surgery; pain during movement (mobilization to sitting position or coughing), with pain scores measured on a NRS (ranging from 0–10) every hour during the PACU stay (average); pain during admission, reported once daily on a NRS scale (0–10), on POD 0–4 (or day 3 if discharged); analgesic medication—rescue opioids during admission (POD 0–4, or day 3 if discharged), converted to oral morphine equivalents according to a standardized opioid conversion chart23; nausea during admission, reported once daily on a four-point NRS scale (none, light, moderate or severe), on POD 0–4 (or day 3 if discharged); duration of PACU and hospital stay, measured from time of operation until time of discharge; and changes in liver enzymes and function tests (alanine aminotransferase (ALT), prothrombin time, total bilirubin) at baseline (preoperative evaluation) and on POD 1 (all patients), 2 and 3 (major resections).
Adverse events
Adverse events were defined as any event occurring within 40 h after administration of the trial drug, except events not requiring any treatment or action (such as deviant laboratory results), hypotension (MAP below 60 mmHg) and/or tachycardia (heart rate over 120 b.p.m.) in the first 24 h after surgery, pain from the operative area, urinary retention, intraoperative bleeding, and motor function affected during epidural treatment. All adverse event were evaluated by the primary investigator with regard to whether they were related, likely to be related, unlikely to be related, or unrelated to the trial drug, and graded from 1 to 4 (1, mild; 2, moderate; 3, severe; 4, life-threatening, disabling or deadly). All serious adverse events (grade 3–4) were reported and analysed for each group.
Data collection
Data were collected from the EPIC based-electronic medical record and managed using REDCap electronic database hosted at Rigshospitalet24. Variables collected were: demographics (age, sex, weight, height, indication for surgery, co-morbidities, smoking and alcohol habits, medication, abdominal operative history, recent chemotherapy history) and intraoperative data (time of start and end of surgery, time of extubation, duration of hepatic clamping, insertion of drains, amount of anaesthetics and fluids administered, bleeding, blood component therapy, level of epidural insertion, type of incision, procedures performed, including additional (unplanned) procedures). Postoperative data were collected relating to the PACU (time of admission and discharge, vital values (BP, heart rate, oxygen saturation, respiratory rate), pain, nausea, and sedation every 30 min, pain at mobilization every 60 min, and treatments administered—oxygen supply, analgesics, antiemetics, blood component therapy, fluid, vasopressors) and the ward (vital values, pain, nausea and sedation at admission, any complications requiring treatment within 24 h, analgesic and antiemetic treatment until POD 4 or discharge (whichever came first), discharge from hospital). All-cause mortality, readmissions (unplanned and related to surgery), and morbidity (as described in secondary outcomes) were assessed by reference to patient charts 30 days after surgery.
Biomarker measurements (haemoglobin, platelets, creatinine, potassium, sodium, prothrombin time (international normalized ratio), ALT, bilirubin) were collected for all patients at baseline and on POD 1, and also on POD 2–3 after major resections.
Patients were requested to fill out a questionnaire every day on POD 0–4 (or day of discharge, whichever came first) containing the following elements pertaining to the previous 24 h (or interval between surgery until bedtime on POD 0): pain (NRS 0–10) on average and at worst, nausea (none, light, moderate, severe) on average and at worst, vomiting (yes/no), feelings of sadness (yes/no), restlessness (yes/no) or fatigue (yes/no), and quality of sleep (good, trouble falling asleep, frequent awakenings, no sleep).
All data were validated by double entry; the data monitoring committee performed random sample data monitoring on all patients and full data set monitoring on 20 per cent.
Statistical analysis
The sample size calculation was based on internal audit and previous reports of a 40 per cent complication rate in the PACU. An assumed reduction in complication rate in the PACU from 40 to 20 per cent in the intervention group required 174 patients (87 in each group), with a two-tailed 5 per cent significance level and a power of 80 per cent.
Categorical parameters are presented as number (per cent) and were compared across groups by Fisher’s exact test. Continuous measures are reported as mean(s.d.) or median (i.q.r.) and were compared between groups using independent-samples t test or Mann–Whitney/Wilcoxon rank-sum test (MWW test) according to the distribution of data determined by inspection of histograms. The primary outcome (composite and components) was compared using Fisher’s exact test, and absolute and relative effect size was calculated with 95 per cent confidence intervals. Secondary outcomes were compared using Fisher’s exact test (morbidity, complications within 24 h, readmissions) or MWW test (duration of stay, pain during movement, cumulative rescue opioids).
The effects of the intervention on changes in ALT, prothrombin time, and bilirubin from baseline to postoperative values between groups were assessed using a constrained linear mixed model with an unstructured co-variance pattern using the nlme package in R (R Foundation for Statistical Computing, Vienna, Austria).
Pain and nausea during admission were repeated measurements but, as data were missing not at random, outcomes were compared between groups day by day using the MWW test.
Data were analysed as intention to treat and all available data were used. Per-protocol analyses were undertaken to test for any important differences.
All analyses were performed with R version 3.6.2 and RStudio® version 1.2.5001 (Boston, MA, USA).
Results
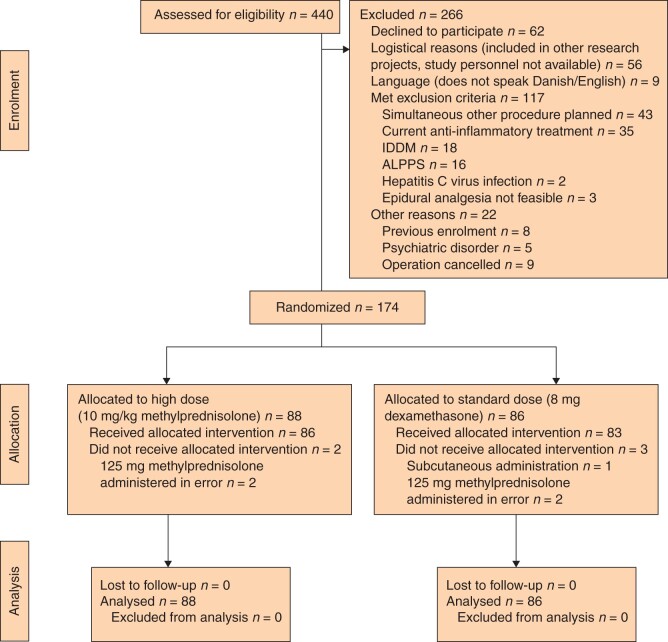
Of 440 patients screened, 174 were included and allocated to the either HD (10 mg/kg methylprednisolone; mean dose 843 mg) or SD (8 mg dexamethasone) group (Fig. 1). Four patients did not receive the allocated intervention owing to a protocol violation and one patient had a subcutaneous injection. All randomized patients were included in the intention-to-treat analysis (presented here) and per-protocol analysis was undertaken for all patients who received the intervention as allocated.
Fig. 1.
CONSORT flow chart for trial
IDDM, insulin-dependent diabetes mellitus; ALPPS, associating liver partitition and portal vein ligation for staged hepatectomy.
Patients were comparable at baseline (Tables 1 and 2). Mean(s.d.) age was 65(12) years, 67 per cent were men, and 30 per cent of the patients had no serious co-morbidities other than the indication for surgery. The most frequent indication for surgery was colorectal metastatic disease (70 per cent), and the operation most often performed was non-anatomical resection (24 per cent). Of the excluded patients, 56 per cent were men and mean age was 64(13) years.
Table 1.
Preoperative characteristics
| High-dose group (n = 88) | Standard-dose group (n = 86) | |
|---|---|---|
| Age (years)* | 65.2(11.2) | 64.4(12.0) |
| Sex ratio (M : F) | 61 : 27 | 56 : 30 |
| BMI (kg/m2)* | 27.4(5.0) | 27.9(5.5) |
| Smoking status | ||
| Former | 43 (48.9) | 46 (53.5) |
| Current | 18 (20.5) | 16 (18.6) |
| Alcohol use (units/week) † | ||
| ≤ 7 (F) or ≤ 14 (M) | 55 (62.5) | 46 (53.5) |
| > 7 (F) or > 14 (M) | 14 (15.9) | 17 (19.8) |
| No co-morbidities | 27 (30.7) | 28 (32.6) |
| Cardiac disease ‡ | 46 (52.3) | 38 (44.2) |
| Chronic pulmonary disease § | 10 (11.4) | 12 (14.0) |
| Non-insulin-dependent diabetes | 11 (12.5) | 11 (12.8) |
| Cirrhosis | 7 (8.0) | 9 (10.5) |
| Depression | 3 (3.4) | 4 (4.7) |
| Concomitant cancer (other) | 6 (6.8) | 6 (7.0) |
| ASA fitness grade | ||
| I | 2 (2.3) | 1 (1.2) |
| II | 41 (46.6) | 42 (48.8) |
| III | 45 (51.1) | 43 (50.0) |
| Indication for surgery | ||
| Colorectal metastases | 63 (71.6) | 58 (67.4) |
| Non-colorectal metastases | 3 (3.4) | 6 (7.0) |
| Hepatocellular carcinoma | 10 (11.4) | 10 (11.6) |
| Cholangiocellular carcinoma | 6 (6.8) | 3 (3.5) |
| Gallbladder cancer | 0 (0.0) | 1 (1.2) |
| Benign disease | 3 (3.4) | 8 (9.3) |
| Other | 3 (3.4) | 0 (0.0) |
| Previous abdominal operation(s) | 57 (64.8) | 50 (58.1) |
| Stratified to major resection (≥ 3 segments) | 22 (25.0) | 22 (25.6) |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.). †Based on Danish Health Agency recommendations of maximum 7 units per week for women and 14 units per week for men. ‡Diagnosed with and receiving medication for any chronic cardiac disease, including arrythmias, hypertension and/or hypercholesterolaemia. §Chronic obstructive bronchitis, emphysema and/or asthma.
Table 2.
Operative characteristics
| High-dose group (n = 88) | Standard-dose group (n = 86) | |
|---|---|---|
| Major procedure performed | 21 (23.9) | 23 (26.7) |
| Primary procedure | ||
| Right/extended right hepatectomy | 5 (5.7) | 13 (15.0) |
| Left hepatectomy | 11 (12.5) | 6 (7.0) |
| Bisegmentectomy | 16 (18.2) | 14 (16.3) |
| Segmentectomy | 11 (12.5) | 9 (10.5) |
| Non-anatomical resection(s) | 20 (22.7) | 21 (24.4) |
| Radiofrequency ablation | 8 (9.1) | 14 (16.3) |
| Exploratory laparotomy | 11 (12.5) | 4 (4.7) |
| Ligation of portal vein | 2 (2.3) | 2 (2.3) |
| Other‡ | 4 (4.5) | 3 (3.5) |
| Other procedure(s) | ||
| Cholecystectomy | 43 (48.9) | 39 (45.3) |
| Radiofrequency ablation | 19 (21.6) | 21 (24.4) |
| Segmentectomy | 5 (5.7) | 1 (1.2) |
| Non-anatomical resection(s) | 14 (15.9) | 14 (16.3) |
| Biopsy | 5 (5.7) | 3 (3.5) |
| Other | 8 (9.1) | 7 (8.1) |
| Duration of operation (min)* | 163.5 (73.6) | 161.8(65.7) |
| Interval from end of operation to extubation (min)* | 13.6 (6.9) | 14.6(7.7) |
| Blood loss † | 503 (216–1173) | 568 (203–989) |
| Abdominal drain | 46 (52.3) | 41 (47.7) |
| Blood transfusion | 13 (14.8) | 10 (11.6) |
| Hepatic clamping | 41 (46.6) | 35 (40.7) |
| Ischaemia time (min) † | 30 (14.5–52) | 28.5 (15–45) |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.) and †median (i.q.r.). ‡Biopsy (4 patients), colectomy/resection (2), hysterectomy/bilateral salpingo-oophorectomy and colonic resection (1).
A total of 109 participants (63 per cent) experienced a complication in the PACU according to the definition of the primary outcome. A complication in the PACU occurred in 51 patients (58 per cent) in the HD group and 58 (67 per cent) in the SD group (absolute reduction 9.5 per cent; risk ratio (RR) 0.86, 95 per cent c.i. 0.68 to 1.08; P = 0.213). Results for the separate components of the primary outcome are shown in Table 3. There were no invasive respiratory treatments, and no patients were transferred to the ICU. The most frequent complication was moderate–severe pain (62 patients), and the median cumulative rescue opioid consumption in the PACU (outcome defined a posteriori) was 11 (i.q.r. 0–30) mg in the SD group versus 12 (0–30) mg in the HD group (P = 0.900). The median pain score on the NRS during movement was 1 (i.q.r. 0–3) in the SD group versus 0 (0–1) in the HD group (P = 0.132). However, this outcome measure was missing for 46 and 39 patients in the SD and HD groups respectively. Per-protocol analyses (excluding patients who did not receive the study drug) also showed no significant differences between allocation groups (data not shown). A post hoc-defined per-protocol analysis of the primary outcome, including only the patients who underwent liver surgery (excluding those who were inoperable or who had radiofrequency ablation only) was performed. In this analysis, 39 patients (59 per cent) in the HD group and 43 (67 per cent) in the SD group had a complication in the PACU, with no statistically significant difference between groups (RR 0.88, 0.68 to 1.15; P = 0.368).
Table 3.
Primary outcome
| High-dose group | Standard-dose group | Risk ratio* | Absolute risk reduction (%)* | P † | |
|---|---|---|---|---|---|
| (n = 88) | (n = 86) | ||||
| No. of patients with a complication in PACU, according to definition of primary outcome | 51 (57.95) | 58 (67.44) | 0.86 (0.68, 1.08) | –9.5 (–23.8, 4.8) | 0.213 |
| Pain | 26 (29.55) | 36 (41.86) | 0.71 (0.47, 1.06) | –12.3 (–26.4, 1.8) | 0.113 |
| Circulatory events | 31 (35.23) | 29 (33.72) | 1.05 (0.69, 1.57) | 1.5 (–12.6, 15.6) | 0.874 |
| Postoperative nausea and vomiting | 7 (7.95) | 12 (13.95) | 0.57 (0.24, 1.38) | –6 (–15.3, 3.3) | 0.232 |
| Respiratory events | 10 (11.36) | 6 (6.98) | 1.63 (0.62, 4.29) | 4.4 (–4.2, 12.9) | 0.433 |
| Sedation | 0 (0) | 1 (1.16) | – | –1.2 (–3.4, 1.1) | 0.494 |
Values in parentheses are percentages unless indicated otherwise; *values in parentheses are 95 per cent confidence intervals. PACU, postanaesthesia care unit. †Fisher’s exact test.
There were no deaths within 30 days. There were no significant differences in duration of stay, complications in the first 24 h, 30-day readmissions or 30-day morbidity, including infectious complications, between allocation groups (Tables 4 and 5). The only notable, albeit non-statistically significant difference, was that more patients in the HD group experienced bile leak (7 versus 1; RR 6.84, 0.86 to 54.44; P = 0.064).
Table 4.
Duration of hospital stay, complications, readmissions, and wound infections
| High-dose group | Standard-dose group | Risk ratio † | P ‡ | |
|---|---|---|---|---|
| (n = 88) | (n = 86) | |||
| Duration of PACU stay (min)* | 202.5 (142.5–320.2) | 185.5 (156.0–224.5) | 0.232§ | |
| Duration of hospital stay (h)* | 97.7 (76.7–147.5) | 101.7 (93.2 –144.0) | 0.160§ | |
| Complications in ward during first 24 h | 23 (26.1) | 29 (33.7) | 0.77 (0.49, 1.23) | 0.346 |
| Readmission within 30 days | 11 (12.5) | 13 (15.1) | 0.83 (0.39, 1.74) | 0.665 |
| Wound infection within 30 days | 6 (6.8) | 7 (8.1) | 0.84 (0.29, 2.39) | 0.780 |
Values in parentheses are percentages unless indicated otherwise; *values are median (i.q.r.) and †values in parentheses are 95 per cent confidence intervals. PACU, postanaesthesia care unit. ‡Fisher’s exact test, except §Mann–Whitney/Wilcoxon rank-sum test.
Table 5.
30-day morbidity
| High-dose group | Standard-dose group | Risk ratio* | Absolute risk reduction (%)* | P ‡ | |
|---|---|---|---|---|---|
| (n = 88) | (n = 86) | ||||
| Overall 30-day morbidity** | 19 (21.6) | 19 (22.1) | 0.98 (0.56, 1.72) | –0.5 (–12.8, 11.8) | 1.000 |
| Abdominal collection (drainage) | 6 (6.8) | 3 (3.5) | 1.96 (0.51, 7.57) | 3.3 (–3.2, 9.9) | 0.496 |
| Ascites (drainage or prolonged use of drain) | 3 (3.4) | 6 (7.0) | 0.49 (0.13, 1.89) | –3.6 (–10.2, 3.0) | 0.327 |
| Infection (systemic) | 5 (5.7) | 4 (4.7) | 1.22 (0.34, 4.40) | 1.0 (–5.5, 7.6) | 1.000 |
| Reoperation (under general anaesthesia, other than fascial dehiscence) | 4 (4.6) | 4 (4.7) | 0.98 (0.25, 3.78) | –0.1 (–6.3, 6.1) | 1.000 |
| Fascial dehiscence (operated) | 3 (3.4) | 5 (5.8) | 0.59 (0.15, 2.38) | –2.4 (–8.6, 3.8) | 0.498 |
| Bile leak | 7 (7.95) | 1 (1.16) | 6.84 (0.86, 54.44) | 6.8 (–0.7, 12.9) | 0.064 |
| Pleural effusion (drainage) | 2 (2.3) | 2 (2.3) | 0.98 (0.14, 6.78) | –0.05 (–4.5, 4.4) | 1.000 |
| Bleeding (gastrointestinal) | 0 (0) | 2 (2.3) | – | –2.3 (–5.5, 0.9) | 0.243 |
| Clavien-Dindo grade † | 0.109 | ||||
| I | 5 (5.7) | 8 (9.3) | |||
| II | 2 (2.3) | 0 (0) | |||
| IIIa | 6 (6.8) | 2(2.3) | |||
| IIIb | 6 (6.8) | 9 (10.5) |
Values in parentheses are percentages unless indicated otherwise; *values in parentheses are 95 per cent confidence intervals. Post hoc classification; for patients with more than one complication, the highest grade is reported. **Not reported in the table due to low or no numbers: (n = 1) bowel obstruction (standard-dose group), perforated bowel (standard-dose group), (n = 0): liver failure, lung embolus, deep venous thrombosis, acute myocardial infarction, transitory cerebral ischaemia, apoplexy. ‡Fisher’s exact test.
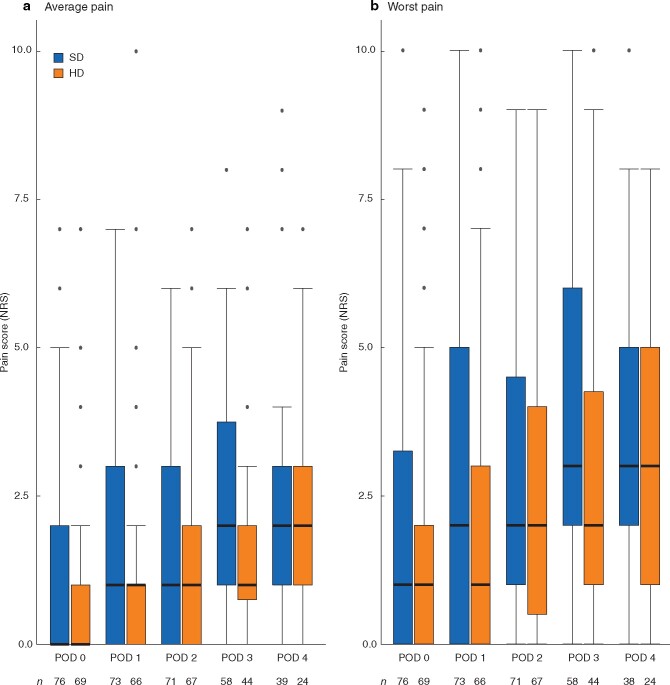
Pain scores on average and at worst, and presence of nausea during the first 4 postoperative days are presented in Fig. 2, Tables S2, and S3. The number of patients with nausea during admission decreased over time in both groups, most notably in the HD group (Table S3). Pain both on average and at worst increased in both groups, reaching a maximum on POD 3 (when the epidural was removed) and further on POD 4 in the HD group. After accounting for repeated measurements, there were no statistically significant differences between groups.
Fig. 2.
Pain during admission
a Average and b worst pain. Median value (bold line), i.q.r. (box), and range (error bars) excluding outliers (dots) are shown. Number of observations each day for each group is indicated below bars. SD, standard dose; HD, high dose; NRS, numerical rating scale; POD, postoperative day.
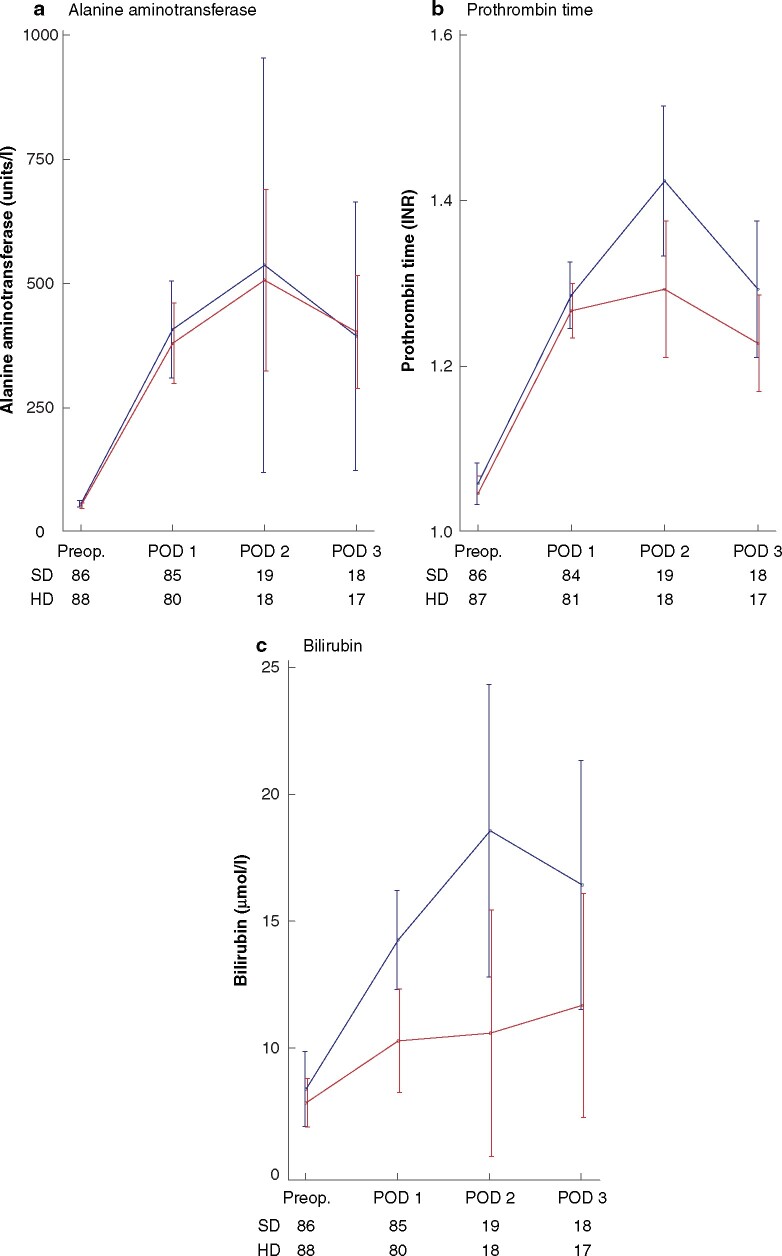
Changes in ALT, prothrombin time, and bilirubin level from baseline are shown in Fig. 3. There was an overall significant effect on prothrombin time in favour of the HD group (P = 0.024) and bilirubin but not ALT level (P = 0.336). Statistically significant differences occurred on POD 2 (prothrombin time and bilirubin) and POD 3 (bilirubin); the latter applies only to patients undergoing major resections.
Fig. 3.
Alanine aminotransferase, prothrombin time, and bilirubin levels measured before surgery and on postoperative day 1 (all patients) and postoperative day 2–3 (major resections only)
a Alanine aminotransferase, b prothrombin time, and c bilirubin. Values are mean(s.d.). Number of observations each day for each group is indicated below graph. SD, standard dose; HD, high dose; preop., before surgery (baseline); POD, postoperative day; INR, international normalized ratio. *P < 0.050, †P < 0.001 versus SD (Likelihood-ratio test).
Some 49 patients (43 per cent) in the SD group and 48 (45 per cent) in the HD group had an adverse event during the first 40 h after administration of trial drug (difference –2.4 (95 per cent c.i. –17.2 to 12.3) per cent). Most of the adverse events documented were pain in the right shoulder during the PACU stay. There were three serious adverse events, one in the SD group (variceal bleeding) and two in the HD group (1 patient with an iatrogenic bile duct lesion, reoperated within 40 h, and one patient with suspected a bile lesion who underwent endoscopic retrograde cholangiopancreatography). There were no adverse reactions, serious adverse reactions or serious unexpected suspected adverse reactions.
Discussion
A preoperative high dose of intravenous methylprednisolone (10 mg/kg) before open liver surgery did not reduce the number of patients with a complication requiring treatment in the PACU compared with 8 mg dexamethasone. At best, the difference was an absolute reduction of 24 per cent, and at worst an increment of 5 per cent. The most frequently occurring complication was pain and, although not statistically significant, patients in the HD group were less likely (at best 26 per cent less likely, and at worst 2 per cent more likely) to experience this complication in the PACU. However, there were no significant differences in pain scores or opioid consumption during admission. Studies of other procedures have shown conflicting results on the analgesic effects of GCs, with some reporting a reduction in postoperative pain scores25–27, and others no difference between groups28–30. Apart from explaining the different conclusions in terms of small study populations, differences in doses, outcome measures, and statistical analyses, there are most likely also procedure-specific differences in the magnitude of the inflammatory response and the mechanisms of pain.
The second most frequent complication in the PACU was circulatory events, mainly hypotension treated with noradrenaline, potentially related to the relative functional hypovolaemia from the epidural and restrictive intraoperative fluid regimen to reduce CVP and thus bleeding from the liver resection. Few patients experienced PONV in the PACU, reflecting that all patients received the PONV prophylactic dose of GCs and ondansetron. Respiratory events were also uncommon, occurring most frequently in patients with preoperative pulmonary disease requiring supplementary oxygen at higher levels.
Previous randomized studies6,11–13 of liver surgery, in which methylprednisolone was administered in doses ranging from 500 mg to 30 mg/kg, showed an effect of high-dose GCs in reducing levels of inflammatory markers such as C-reactive protein, interleukin (IL) 6, IL-8, and IL-10, and liver function markers such as AST and bilirubin, and a reduced length of stay6,11, but no differences in clinical (secondary) outcomes such as complications. The most recent, and until now the largest, RCT14 was the only study to report a clinical primary outcome, namely the 90-day morbidity rate. The study investigated the total complication rate 90 days after surgery in 151 patients undergoing major hepatic resections who received either a preoperative dose of 500 mg methylprednisolone or placebo. A lower complication rate in the GC group was reported and, although the study was probably underpowered, there was a signal towards fewer overall complications (24 patients (31 per cent) in the GC group versus 35 (47 per cent) in the placebo group). However, the reported decrease in surgical-site infections did not hold statistical power when corrected for multiple comparisons. The duration of hospital stay in previous studies ranged from 6 to 19 days, whereas median hospital stay in the present study was 4 days, leaving little room for improvement. In this study, there was an overall significant positive effect on prothrombin time and bilirubin level on POD 2, and bilirubin on POD 3, in the HD group. The data were, however, only from major resections, and the confidence intervals were wide, so this result should be interpreted with caution. Furthermore, there was a difference in the extent of major resections between groups, which adds to the uncertainty of the results. There were no apparent clinical consequences of the differences, and the increase was transient in both groups. Inflammatory markers were not measured, as the effects of GCs in reducing these have already been shown in numerous studies15,18,31,32. There were no statistically significant differences between groups in adverse events, wound infections, wound/fascial dehiscence or 30-day morbidity, but an indication of increased risk of bile leak was noted in the HD group. However, the present study was not powered for morbidity outcomes, and, although the recently published RCT14 did not report an increased risk of bile leak in the GC group, the number of patients is actually larger in present study. Thus, the observation of increased bile leakage still calls for caution, as the previous RCT14 was probably also underpowered for morbidity outcomes as the sample size calculation was based on a more than 75 per cent relative decrease in complication rates, whereas the observed decrease was around 40 per cent.
As a double-blinded RCT in a clinical setting with an ERAS regimen in place, this study has high internal validity. The generalizability, however, is limited by the exclusion criteria and the single-centre design. The primary outcome was a composite based on different complications arising in the PACU, and the study was not powered to assess the impact of the intervention on each specific complication. The definition of the primary outcome was based on the widely used postanaesthesia recovery score (modified Aldrete criteria). These criteria are used to observe and risk assess patients after anaesthesia in order to react quickly to complications, and to assess when patients are stable enough for transfer to the surgical ward. Studies33–35 have questioned the validity and reliability of several of the assessment variables in the PACU discharge criteria, and modifications are continuously being evaluated. It is, however, currently the most widely used postanaesthesia scoring system and so basing the primary outcome on this score allows the findings to have an impact on clinical practice.
As there are no studies of the early clinical effects of a very high dose of preoperative GCs, the sample size calculation was based on assumptions. In hindsight, these were perhaps optimistic, and it is debatable whether the minimal clinically important difference is smaller. Nonetheless, if a potent drug is to be implemented to enhance recovery, it should be effective enough for its use to be justified. The greatest specific complication difference between groups was in the occurrence of moderate–severe pain in the PACU; if the true difference is around a 12 per cent absolute reduction (25 per cent relative reduction), without reducing opioid treatment, duration of stay or pain scores during admission, it is of questionable clinical importance in the authors’ opinion.
There were no missing data for the primary outcome. Data on pain during movement in the PACU were not missing at random, but were not available when patients were too drowsy or had motor function too affected by the epidural analgesia to participate; this variable was analysed after listwise deletion of patients with missing data. Missing data for pain and nausea during admission can be explained by a few patients declining to participate, some patients forgetting to answer or to hand in the questionnaires, and data being missing on day 4 for patients who had already been discharged. As patients who are discharged early are less likely to experience pain and nausea, these data were also assumed not to be missing at random, and were analysed in the same way.
This study included an active comparator group receiving 8 mg dexamethasone instead of a placebo group as dexamethasone in the range 4–8 mg has been shown to reduce PONV primarily, but also pain and opioid consumption across a variety of surgical procedures20,36 and is the recommended standard treatment for PONV20.
This study has shown the importance of testing interventions by specific procedure, under optimized clinical settings, and with clinically relevant outcomes. Preoperative high-dose GCs modulate the immune response in ways that are only partially understood37 and, until there is meaningful clinical evidence, it is probably best to err on the side of caution. Based on the present data, there is currently no evidence for superiority of a dose of preoperative GCs higher than 8 mg dexamethasone for prevention of acute postoperative complications in patients undergoing open liver resection.
Funding
This study was supported financially by the Research Fund at Rigshospitalet and Departments of Anaesthesiology and Gastrointestinal Surgery and Transplantation, Centre for Cancer and Organ Diseases, Rigshospitalet Copenhagen University Hospital, Denmark.
Supplementary Material
Acknowledgements
J. L. Forman (Section of Biostatistics, Department of Public Health, University of Copenhagen) is acknowledged for statistical consultancy, analysis, and interpretation of the repeated measurements; M. I. V. Søgaard and E. Sigvardt for help with preparing the study drug; and the staff at department 2043 and 2123, Rigshospitalet, for help with coordinating recruitment of patients and preparation of study drugs.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Contributor Information
K J Steinthorsdottir, Department of Anaesthesiology, Centre for Cancer and Organ Diseases, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark; Section of Surgical Pathophysiology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
H N Awada, Department of Anaesthesiology, Centre for Cancer and Organ Diseases, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark.
N A Schultz, Department of Gastrointestinal Surgery and Transplantation, Centre for Cancer and Organ Diseases, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark.
P N Larsen, Department of Gastrointestinal Surgery and Transplantation, Centre for Cancer and Organ Diseases, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark.
J G Hillingsø, Department of Gastrointestinal Surgery and Transplantation, Centre for Cancer and Organ Diseases, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark.
Ø Jans, Department of Anaesthesiology, Centre for Cancer and Organ Diseases, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark.
H Kehlet, Section of Surgical Pathophysiology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
E K Aasvang, Department of Anaesthesiology, Centre for Cancer and Organ Diseases, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark.
References
- 1. Schultz NA, Larsen PN, Klarskov B, Plum LM, Frederiksen HJ, Kehlet H et al. Second generation of a fast-track liver resection programme. World J Surg 2018;42:1860–1866. [DOI] [PubMed] [Google Scholar]
- 2. Mayo SC, Heckman JE, Shore AD, Nathan H, Parikh AA, Bridges JFP et al. Shifting trends in liver-directed management of patients with colorectal liver metastasis: a population-based analysis. Surgery 2011;150:204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayo SC, Pulitano C, Marques H, Lamelas J, Wolfgang CL, Saussure WD et al. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg 2013;216:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mavros MN, Jong MD, Dogeas E, Hyder O, Pawlik TM. Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg 2013;100:711–718. [DOI] [PubMed] [Google Scholar]
- 5. Kabir T, Syn NL, Tan ZZX, Tan HJ, Yen C, Koh YX et al. Predictors of post-operative complications after surgical resection of hepatocellular carcinoma and their prognostic effects on outcome and survival: a propensity-score matched and structural equation modelling study. Eur J Surg Oncol 2020;46:1756–1765. [DOI] [PubMed] [Google Scholar]
- 6. Aldrighetti L, Pulitanò C, Arru M, Finazzi R, Catena M, Soldini L et al. Impact of preoperative steroids administration on ischemia–reperfusion injury and systemic responses in liver surgery: a prospective randomized study. Liver Transpl 2006;12:941–949. [DOI] [PubMed] [Google Scholar]
- 7. Steinthorsdottir KJ, Kehlet H, Aasvang EK. Surgical stress response and the potential role of preoperative glucocorticoids on post-anesthesia care unit recovery. Minerva Anestesiol 2017;83:1324–1331. [DOI] [PubMed] [Google Scholar]
- 8. Pitter SET, Kehlet H, Hansen CP, Bundgaard-Nielsen M, Storkholm J, Aasvang EK. Persistent severe post-operative hypotension after pancreaticoduodenectomy is related to increased inflammatory response. Acta Anaesthesiol Scand 2020;64:455–463. [DOI] [PubMed] [Google Scholar]
- 9. Li H, Wei Y, Li B. Preoperative steroid administration in liver resection: a systematic review and meta-analysis. Hepatogastroenterology 2012;60:160–169. [DOI] [PubMed] [Google Scholar]
- 10. Orci LA, Toso C, Mentha G, Morel P, Majno PE. Systematic review and meta-analysis of the effect of perioperative steroids on ischaemia–reperfusion injury and surgical stress response in patients undergoing liver resection. Br J Surg 2013;100:600–609. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt SC, Hamann S, Langrehr JM, Höflich C, Mittler J, Jacob D et al. Preoperative high-dose steroid administration attenuates the surgical stress response following liver resection: results of a prospective randomized study. J Hepatobiliary Pancreat Surg 2007;14:484–492. [DOI] [PubMed] [Google Scholar]
- 12. Yamashita Y, Shimada M, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K et al. Effects of preoperative steroid administration on surgical stress in hepatic resection. Arch Surg 2001;136:328–333. [DOI] [PubMed] [Google Scholar]
- 13. Muratore A, Ribero D, Ferrero A, Bergero R, Capussotti L. Prospective randomized study of steroids in the prevention of ischaemic injury during hepatic resection with pedicle clamping. Br J Surg 2003;90:17–22. [DOI] [PubMed] [Google Scholar]
- 14. Bressan AK, Isherwood S, Bathe OF, Dixon E, Sutherland FR, Ball CG. Preoperative single-dose methylprednisolone prevents surgical site infections after major liver resection. Ann Surg 2020. Dec 18;Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 15. de la Motte L, Kehlet H, Vogt K, Nielsen CH, Groenvall JB, Nielsen HB et al. Preoperative methylprednisolone enhances recovery after endovascular aortic repair. Ann Surg 2014;260:540–549. [DOI] [PubMed] [Google Scholar]
- 16. Vignali A, Palo SD, Orsenigo E, Ghirardelli L, Radaelli G, Staudacher C. Effect of prednisolone on local and systemic response in laparoscopic vs. open colon surgery. Dis Colon Rectum 2009;52:1080–1088. [DOI] [PubMed] [Google Scholar]
- 17. Aminmansour B, Khalili HA, Ahmadi J, Nourian M. Effect of high-dose intravenous dexamethasone on postlumbar discectomy pain. Spine (Phila Pa 1976) 2006;31:2415–2417. [DOI] [PubMed] [Google Scholar]
- 18. Sato N, Koeda K, Ikeda K, Kimura Y, Aoki K, Iwaya T et al. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg 2002;236:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliveira GD, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain. Anesthesiology 2011;115:575–588. [DOI] [PubMed] [Google Scholar]
- 20. Oliveira GD, Castro-Alves LJS, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg 2013;116:58–74. [DOI] [PubMed] [Google Scholar]
- 21. Schultz NA, Larsen PN, Klarskov B, Plum LM, Frederiksen HJ, Christensen BM et al. Evaluation of a fast-track programme for patients undergoing liver resection. Br J Surg 2012;100:138–143. [DOI] [PubMed] [Google Scholar]
- 22. Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg 1970;49:924–934. [PubMed] [Google Scholar]
- 23. Svendsen K, Borchgrevink P, Fredheim O, Hamunen K, Mellbye A, Dale O. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med 2011;25:725–732. [DOI] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bjerregaard LS, Jensen PF, Bigler DR, Petersen RH, Møller-Sørensen H, Gefke K et al. High-dose methylprednisolone in video-assisted thoracoscopic surgery lobectomy: a randomized controlled trial. Eur J Cardiothorac Surg 2018;53:209–215. [DOI] [PubMed] [Google Scholar]
- 26. Lunn TH, Kristensen BB, Andersen LØ, Husted H, Otte KS, Gaarn-Larsen L et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth 2011;106:230–238. [DOI] [PubMed] [Google Scholar]
- 27. Jensen KK, Brøndum TL, Leerhøy B, Belhage B, Hensler M, Arnesen RB et al. Preoperative, single, high-dose glucocorticoid administration in abdominal wall reconstruction: a randomized, double-blinded clinical trial. Surgery 2020;167:757–764. [DOI] [PubMed] [Google Scholar]
- 28. Kleif J, Hauge CI, Vilandt J, Gögenur I. Randomized clinical trial of preoperative high-dose methylprednisolone on postoperative pain at rest after laparoscopic appendectomy. Anesth Analg 2018;126:1712–1720. [DOI] [PubMed] [Google Scholar]
- 29. Aabakke AJM, Holst LB, Jørgensen JC, Secher NJ. The effect of a preoperative single-dose methylprednisolone on postoperative pain after abdominal hysterectomy: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2014;180:83–88. [DOI] [PubMed] [Google Scholar]
- 30. Steinthorsdottir KJ, Awada HN, Abildstrøm H, Kroman N, Kehlet H, Kvanner Aasvang E. Dexamethasone dose and early postoperative recovery after mastectomy. Anesthesiology 2020;132:678–691. [DOI] [PubMed] [Google Scholar]
- 31. Richardson AJ, Laurence JM, Lam VWT. Use of pre-operative steroids in liver resection: a systematic review and meta-analysis. HPB (Oxford) 2014;16:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McSorley ST, Horgan PG, McMillan DC. The impact of preoperative corticosteroids on the systemic inflammatory response and postoperative complications following surgery for gastrointestinal cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2016;101:139–150. [DOI] [PubMed] [Google Scholar]
- 33. Phillips NM, Street M, Kent B, Haesler E, Cadeddu M. Post-anaesthetic discharge scoring criteria: key findings from a systematic review. Int J Evid Based Healthc 2013;11:275–284. [DOI] [PubMed] [Google Scholar]
- 34. Aasvang EK, Jørgensen CC, Laursen MB, Madsen J, Solgaard S, Krøigaard M et al. Safety aspects of postanesthesia care unit discharge without motor function assessment after spinal anesthesia: a randomized, multicenter, semiblinded, noninferiority, controlled trial. Anesthesiology 2017;126:1043–1052. [DOI] [PubMed] [Google Scholar]
- 35. Bjerregaard LS, Hornum U, Troldborg C, Bogoe S, Bagi P, Kehlet H. Postoperative urinary catheterization thresholds of 500 versus 800 ml after fast-track total hip and knee arthroplasty. Anesthesiology 2016;124:1256–1264. [DOI] [PubMed] [Google Scholar]
- 36. Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth 2013;110:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ganio EA, Stanley N, Lindberg-Larsen V, Einhaus J, Tsai AS, Verdonk F, Culos A et al. Preferential inhibition of adaptive immune system dynamics by glucocorticoids in patients after acute surgical trauma. Nat Commun 2020;11:3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.