Abstract
Purpose
In recent years, less options are available for treating carbapenem-resistant Acinetobacter baumannii and carbapenem-resistant Pseudomonas aeruginosa. The present study investigates the susceptibility rates to imipenem/relebactam for the treatment of intra-abdominal infections (IAIs), respiratory tract infections (RTIs) and urinary tract infections (UTIs) caused by A. baumannii and P. aeruginosa in China.
Patients and Methods
A total of 1886 P. aeruginosa and 1889 A. baumannii isolates were collected in 21 centers (7 regions) as a part of the global SMART surveillance program between 2015 and 2018. Antimicrobial susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) recommendations using the broth microdilution methodology at Peking Union Medical College Hospital.
Results
For P. aeruginosa, overall susceptibility rates to imipenem/relebactam were 84.2% at a CLSI breakpoint of ≤2 mg/L compared to 55.7% for imipenem. Susceptibility rates of imipenem-non-susceptible P. aeruginosa to imipenem/relebactam were 64.4% and for multidrug-resistance (MDR) P. aeruginosa susceptibility rates were increased from 25.2% for imipenem to 65.8% for imipenem/relebactam. The susceptibilities of imipenem-non-susceptible and MDR P. aeruginosa strains were similarly restored by imipenem/relebactam in non-ICU and ICU wards. The rate of imipenem-non-susceptibilities A. baumannii isolates was 79.0%, whereas the MDR rate was 81.9%. Relebactam did not change the susceptibilities of imipenem-non susceptible or MDR A. baumannii isolates.
Conclusion
Imipenem/relebactam provides a therapy option to treat infections caused by MDR or imipenem-non-susceptible P. aeruginosa but not A. baumannii infections in China.
Keywords: carbapenem-resistance, extended-spectrum β-lactamase, multidrug-resistance, β-lactamase inhibitor, carbapenemase
Introduction
Pseudomonas aeruginosa and Acinetobacter baumannii are included in the “ESKAPE” pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, P. aeruginosa and Enterobacter species) and have been reported to be the major cause of nosocomial antimicrobial-resistant infections.1
A. baumannii is a Gram-negative bacterium, which can become an opportunistic pathogen for humans with mortality rates of up to 35%2 and is a common cause of nosocomial pneumonia in intensive care units (ICUs).3 The rate of carbapenem- and ampicillin-sulbactam-resistance in nosocomial bloodstream infections was 25% between 2006 and 2009 in the USA,4 but susceptibility of multidurg-resistance (MDR) A. baumannii to carbapenems or sulbactam in the USA have been estimated to be only 26% in a more recent study.5 In China, the prevalence of imipenem and extensively drug-resistant A. baumannii strains increased from 13.3% and 11.1% in 2004 to 70.5% and 60.4% in 2014.6
P. aeruginosa is also an opportunistic human pathogen frequently causing nosocomial bloodstream as well as respiratory and urinary tract or wound infections7 and was the sixth most commonly found pathogen in healthcare-associated infections.8 The World Health Organization categorized carbapenem-resistant P. aeruginosa and A. baumannii as critical-priority bacteria within 20 antimicrobial-resistant bacteria species.9
An approach to cope with carbapenem resistance is the combination of β-lactamase inhibitors with β-lactams.10 Relebactam is a non-β-lactam bicyclic diazabicyclooctane β-lactamase inhibitor of class A including Klebsiella pneumoniae carbapenemase (KPC) and class C β-lactamases.11 Relebactam does not inhibit class B metallo-β-lactamases or class D oxacillinase (OXA)-type β-lactamases.12
In 2020, the results of a multicenter, randomized, double-blind trial (RESTORE-IMI 1 Study) that compared the efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections revealed that imipenem/relebactam was an efficacious and well-tolerated therapy option for carbapenem-non-susceptible infections.13 Another randomized, controlled, double-blind Phase 3 trial to evaluate the efficacy and safety of imipenem/relebactam in treating hospital-acquired/ventilator-associated bacterial pneumonia (HABP/VABP) (RESTORE-IMI 2 Study)14 found that imipenem/relebactam was a new treatment option for HABP/VABP, including for high-risk patients. Also, during in vitro tests, relebactam restored the imipenem activity against P. aeruginosa.15–17 The Study for Monitoring Antimicrobial Resistance Trends (SMART) revealed that in the USA that imipenem-relebactam and imipenem susceptibilities were 93.1% and 68.1%, respectively for P. aeruginosa for the treatment of lower respiratory tract infections (RTIs) in 2015, and susceptibility to imipenem/relebactam was 82.2% for MDR isolates, while in 2016 relebactam restored imipenem susceptibility to 80.5% (202/251) of imipenem-nonsusceptible P. aeruginosa isolates.18–20 Other SMART multicenter antimicrobial resistance results also reported for Europe revealed that the rates of susceptibility to imipenem and imipenem/relebactam (minimum inhibitory concentration (MIC) ≤ 4 mg/L) were 69.4% and 92.4%, respectively. Of all MDR isolates, 78.2% were susceptible to imipenem/relebactam and the susceptibility rates to imipenem/relebactam were similar for MDR isolates from lower RTIs (77.8% susceptible), intraabdominal tract infections (IAIs) (80.3%) and urinary tract infections (UTIs) (76.4%) in Europe from 2015 to 2017.21 Based on these results, imipenem/relebactam has been shown to be a promising therapeutic option for treating patients with infections caused by antimicrobial-resistant Gram-negative bacilli in Europe and the USA.
SMART is a worldwide surveillance program for determining the susceptibilities to various antibiotic agents of Gram-negative bacteria isolated from IAIs, UTIs and RTIs. The aim of the present study was to assess the in vitro activity of imipenem/relebactam, imipenem and comparators against P. aeruginosa and A. baumannii strains in China from 2015 to 2018 in order to provide useful reference data for future applications in Chinese clinical practice.
Patients and Methods
Clinical Isolates
In total, 1886 P. aeruginosa and 1889 A. baumannii samples were collected from IAI, lower RTI and UTI specimens collected between 2015 and 2018 from 21 centers in 7 Chinese regions (north, northeast, southwest, central, south, east non-Jiangzhe and east Jiangzhe), with a range of 77 to 250 facultative anaerobic and aerobic Gram-negative bacteria per year per hospital, which were consecutively collected. The collection only included unique bacterial isolates and excluded duplicate isolates. Most of the IAI specimens were obtained from the stomach, gall bladder, small intestine, colon, appendix, pancreas, liver, rectum abscesses or peritoneal fluid. UTI specimens were obtained from the urethra or urine. Most RTI specimens came from sputum, bronchoalveolar lavage, bronchial brushings or thoracentesis. P. aeruginosa and A. baumannii isolates were collected from patients in non-ICU or ICU wards. ICU wards included medicine, pediatric, surgery and general unspecified. Non-ICU wards included the emergency room and general medicine, pediatric and surgery wards.
The isolated bacterial species were initially identified and confirmed as Gram-negative bacilli in local hospital laboratories before being sent to Peking Union Medical College Hospital for re-identification confirmation using MALDITOF MS (Bruker Daltonics, MS, USA). Each ethics committee of a participating hospital gave approval for the protocols employed (Et. Number: S-K238).
Susceptibility of Bacteria to Antimicrobial Agents
Isolates were tested for antimicrobial susceptibility using Trek Diagnostic System (Thermo Fisher Scientific, Oakwood Village, OH, USA) with dehydrated broth microdilution panels according to the most recent clinical and laboratory standards institute (CLSI) guidelines at Peking Union Medical College Hospital. The antimicrobials imipenem/relebactam, imipenem, amikacin, cefepime, ceftazidime, aztreonam, ciprofloxacin, piperacillin/tazobactam and colistin were investigated. Relebactam at a fixed 4 mg/L combined with 2-fold imipenem dilutions was tested.
MICs were tested by using the CLSI breakpoints for the drugs, with the exception of imipenem/relebactam.22 Current FDA MIC break-points for imipenem/relebactam tested against P. aeruginosa (≤ 2 mg/mL, susceptible; 4 mg/mL, intermediate; ≥ 8 mg/mL, resistant) and A. baumannii (≤ 2 mg/mL, susceptible; 4 mg/mL, intermediate; ≥ 8 mg/mL, resistant) were applied in our study.23 MDR of P. aeruginosa was defined as non-susceptibility (intermediate or resistant) to at least 3 of 7 sentinel antimicrobial drugs: amikacin, aztreonam, cefepime, ciprofloxacin, colistin, imipenem and piperacillin/tazobactam.24 The MDR of A. baumannii was defined as non-susceptibility (intermediate or resistant) to at least 3 of 6 sentinel antimicrobial drugs: amikacin, cefepime, ciprofloxacin, colistin, imipenem and piperacillin/tazobactam.24 Trends were assessed for statistical significance using the Cochrane-Armitage test.
Quality control testing (QC) was performed each day of testing using the CLSI recommended American Type Culture Collection (ATCC) in the Chinese SMART surveillance program: E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as QC strains. Data were only included when the quality control test results were in acceptable ranges.
Results and Discussion
General Distribution of P. aeruginosa and A. baumannii from 2015 to 2018
In the present study, the isolates of P. aeruginosa from IAIs, RTIs and UTIs were 21.5%, 65.6% and 12.9%, respectively comprising a total of 1886 strains including 23.4% isolates from ICUs. From 1889 A. baumannii isolates 21.0%, 72.4% and 6.7% were collected from IAIs, RTIs and UTIs, respectively and among them 38.1% were from ICUs.
For P. aeruginosa strains, MDR and imipenem-non-susceptible strains accounted for 44.3% and regarding different organs and clinical departments, RTIs and non-ICUs were the main sources of the collected specimens.
For A. baumannii strains, MDR and imipenem-non-susceptible strains accounted for 81.9% and 79.0%, respectively while the majority of specimens came from IAIs and RTIs collected in ICUs (Supplementary Table 1).
In vitro Activity of Imipenem/Relebactam Against P. aeruginosa and A. baumannii from 2015 to 2018
For P. aeruginosa, we found 84.2% susceptibility rates to imipenem/relebactam at a CLSI breakpoint of ≤ 2 mg/L compared to 55.7% susceptibility rates to imipenem. The MIC90 of imipenem (32 mg/L) was 4-fold higher than that of imipenem/relebactam (8 mg/L) (Table 1). These imipenem susceptibility rates were numerically lower than those in other countries as per published SMART reports, with 73% in the USA/Canada and 66.7% in Europe,25 which reflected the more serious resistant status of P. aeruginosa that China faces. In addition, the China Antimicrobial Surveillance Network (CHINET) Program reported that 63.7% of collected P. aeruginosa isolates were susceptible to imipenem in 2017, higher than our findings. The difference between the two studies is likely derived from the difference of the collected isolates. CHINET strains were obtained from both inpatients and outpatients whereas in our study isolates were only taken from hospitalized patients.26 The susceptibility rates of imipenem-non-susceptible P. aeruginosa and MDR P. aeruginosa to imipenem/relebactam were 64.4% and 65.8%, with susceptibilities to imipenem of 0% and 25.2%, respectively (Table 1). The rate of imipenem susceptibility restoration by relebactam in the present study was somewhat lower than reported values for Europe in 2015 (81.1%),27 in the USA in 2015 (80.5%),20 and in Europe 2015–2017 (75.2%),21 but closer to the global data between 2015–2016 of 70.3%.25
Table 1.
In vitro Activity of Imipenem/Relebactam and Imipenem Against P. Aeruginosa and A. Baumannii Collected in China from 2015 to 2018
| Organism/Antimicrobial Agent | MIC (mg/L) | MIC Interpretation | ||||
|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MIC Range | Susceptible Rate (%) | Intermediate Rate (%) | Resistant Rate (%) | |
| P. aeruginosa (N = 1886) | ||||||
| Imipenem/relebactam | 0.5 | 8 | < 0.6 to > 32 | 84.2 | 5.0 | 10.8 |
| Imipenem | 2 | 32 | < 0.12 to > 32 | 55.7 | 6.2 | 38.1 |
| Imipenem-non-susceptible P. aeruginosa (N = 835) | ||||||
| Imipenem/relebactam | 2 | > 32 | 0.25 to > 32 | 64.4 | 11.1 | 24.4 |
| Imipenem | 16 | > 32 | 4 to > 32 | 0.0 | 13.9 | 86.1 |
| MDR P. aeruginosa (N = 835) | ||||||
| Imipenem/relebactam | 2 | > 32 | < 0.06 to > 32 | 65.8 | 10.2 | 24.1 |
| Imipenem | 16 | > 32 | 0.25 to > 32 | 25.2 | 6.7 | 68.1 |
| A. baumannii (N = 1889) | ||||||
| Imipenem/relebactam | 32 | > 32 | < 0.06 to > 32 | 22.2 | 0.6 | 77.2 |
| Imipenem | 32 | > 32 | < 0.12 to > 32 | 21.0 | 0.5 | 78.6 |
| Imipenem-non-susceptible A. baumannii (N = 1493) | ||||||
| Imipenem/relebactam | 32 | > 32 | 0.25 to > 32 | 1.8 | 0.7 | 97.5 |
| Imipenem | 32 | > 32 | 4 to > 32 | 0.0 | 0.6 | 99.4 |
| MDR A. baumannii (N = 1547) | ||||||
| Imipenem/relebactam | 32 | > 32 | < 0.06 to >32 | 5.3 | 0.7 | 94.1 |
| Imipenem | 32 | > 32 | 0.25 to > 32 | 4.0 | 0.4 | 95.6 |
Abbreviations: MIC, minimum inhibitory concentrations; MDR, multidrug resistance.
Adding relebactam did not improve the activity of imipenem against A. baumannii isolates, the susceptibility rate of imipenem and imipenem/relebactam being 21.0% and 22.2% while the susceptibility rates to imipenem and imipenem/relebactam were only 4.0% and 5.3% for MDR A. baumannii isolates, while relebactam yielded only a 1.8% susceptibility regain in imipenem non-susceptible A. baumannii isolates (Table 1). The results from the 2015 SMART surveillance program, involving 17 European countries, revealed that the overall susceptibility of A. baumannii to imipenem was only 10.1%27 and somewhat lower than found in the present study (21.0%). However, in both studies the imipenem non-susceptibilities could not be restored by relebactam in imipenem non-susceptible A. baumannii isolates, which has also been reported in the USA15 and has been attributed to certain resistance mechanisms of A. baumannii strains,28,29 since a main mechanism of carbapenem resistance in A. baumannii is expression of OXAs.30 The A. baumannii percent susceptibilities to imipenem and imipenem/relebactam were virtually identical (both about 20%), whereas for amikacin they were 16.5–32.7%, indicating amikacin resistance, which has been attributed to aminoglycoside-modifying enzyme expressions in A. baumannii.31
Of the comparator antibiotics, amikacin (89.7% susceptible) demonstrated an in vitro rate of susceptibility ≥ 80% only for P. aeruginosa. However, susceptibility rates were high to colistin in all P. aeruginosa (94.5–94.9%) and A. baumannii (96.1–96.5%) isolates, including imipenem-non- susceptible and MDR isolates (Supplementary Table 2), which might be explained by the fact that in China colistin was used in veterinary medicine only until 2014, probably due to its previously reported serious nephrotoxicity and neurotoxicity, which has limited its clinical use.32
Given that relebactam did not restore susceptibility to imipenem for A. baumannii including MDR A. baumannii, we then focused our further analyses on P. aeruginosa.
MIC Frequency Distribution of Imipenem/Relebactam to P. aeruginosa Including Imipenem-Non-Susceptible P. aeruginosa and MDR P. aeruginosa
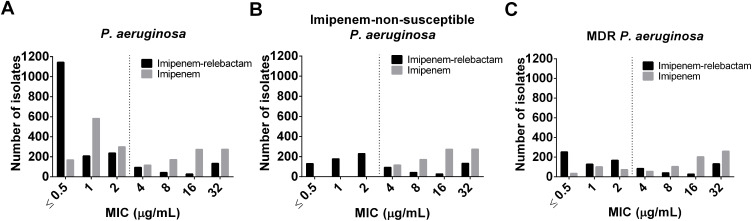
The modal MIC value for imipenem was 1 mg/L (n = 583, 30.9%) and for imipenem/relebactam 0.5 mg/L (n = 1143, 60.6%) (Figure 1A). In addition, 15.6%, 21.3% and 27.5% of imipenem-non-susceptible P. aeruginosa strains were inhibited at a concentration of ≤ 0.5 mg/L, 1 mg/L and 2 mg/L imipenem/relebactam (represented by imipenem concentration), respectively. The modal MiC value for imipenem was 32 mg/L (32.9%) (Figure 1B). For the MDR P. aeruginosa isolates the MIC distributions of imipenem/relebactam were 30.3%, 15.3% and 20.1% at ≤ 0.5 mg/L, 1 mg/L and 2 mg/L, respectively. The modal MICs were 32 mg/L for imipenem and 0.5 mg/L for imipenem/relebactam (Figure 1C).
Figure 1.
Effect of relebactam on MIC distribution of imipenem against: (A) P. aeruginosa isolates (n = 1886); (B) imipenem-non-susceptible P. aeruginosa (n = 835); (C) MDR P. aeruginosa isolates (n = 835). Dashed line represents the FDA identified susceptibility breakpoint of imipenem/relebactam of ≤ 2 mg/mL for P. aeruginosa.
Abbreviations: MIC, minimum inhibitory concentrations; MDR, multidrug resistance.
Susceptibility Changes of P. aeruginosa, MDR P. aeruginosa and Imipenem-Non-Susceptible P. aeruginosa Isolates to Imipenem and Imipenem/Relebactam Obtained from Different Organs (IAIs, UTIs, RTIs) and Clinical Departments (ICU, Non-ICU), Year and Geographic Regions of China
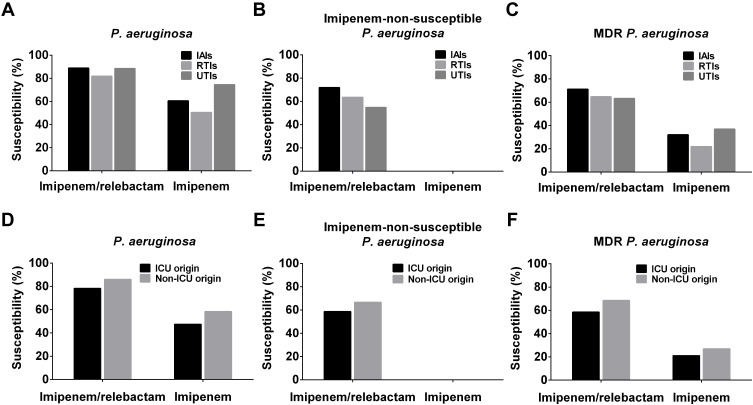
The susceptibility rates of P. aeruginosa isolates from IAIs, RTIs and UTIs against imipenem/relebactam were all greater than 80% and among them the susceptibility rates of P. aeruginosa isolates from IAIs and UTIs were close to 90%. For imipenem-non-susceptible P. aeruginosa isolates, the susceptibility rate of imipenem/relebactam to isolates from IAIs and RTIs were 71.9% and 63.5%, respectively, while for a relatively small number of UTI isolates, the susceptible rate was about 54.8% (Figure 2, Supplementary Table 3).
Figure 2.
Comparison of P. aeruginosa strain susceptibilities. From IAIs, UTIs and RTIs isolated (A) total P. aeruginosa, (B) imipenem-non-susceptible P. aeruginosa and (C) MDR P. aeruginosa strain susceptibilities to imipenem/relebactam, and imipenem. From ICUs and non-ICU departments isolated (D) total P. aeruginosa, (E) imipenem-non-susceptible P. aeruginosa and (F) MDR P. aeruginosa strain susceptibilities to imipenem/relebactam, and imipenem.
Abbreviations: IAIs, intra-abdominal infections; ICU, intensive care unit; MDR, multidrug-resistance; RTIs, respiratory tract infections; UTIs, urinary tract infections.
Imipenem/relebactam possessed high in vitro activity against P. aeruginosa not only for isolates from non-ICU wards (86.0% susceptible) but also against isolates from ICUs (78.2% susceptible). By adding relebactam, the susceptibility to imipenem for imipenem non-susceptible P. aeruginosa strains rose from 0.0% to 58.6% and 66.7% in ICU and non-ICU derived P. aeruginosa isolates, respectively, and that of MDR P. aeruginosa isolates rose from 21.0% to 58.5% in ICU and from 26.7% to 68.5% in non-ICU isolates (Table 2). However, The percentages of imipenem non-susceptible P. aeruginosa and MDR P. aeruginosa isolates were numerically higher in ICU than in non-ICU samples, which is in agreement with previously published reports.33,34
Table 2.
In vitro Activity of Imipenem/Relebactam and Imipenem Against P. Aeruginosa Isolates from ICUs and Non-ICU Wards
| Organism/Antimicrobial Agent | ICU Origin (IAIs + UTIs + RTIs) | Non-ICU Origin (IAIs + UTIs + RTIs) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | S% | R% | MIC90 | N | S% | R% | MIC90 | |
| P. aeruginosa | ||||||||
| Imipenem/relebactam | 441 | 345 (78.2) | 72 (16.3) | 32 | 1445 | 1243 (86.0) | 132 (9.1) | 4 |
| Imipenem | 441 | 209 (47.4) | 208 (47.2) | > 32 | 1445 | 842 (58.3) | 511 (35.4) | 32 |
| Imipenem-non-susceptible P. aeruginosa | ||||||||
| Imipenem/relebactam | 232 | 136 (58.6) | 72 (31.0) | > 32 | 603 | 402 (66.7) | 132 (21.9) | 32 |
| Imipenem | 232 | 0 (0.0) | 208 (89.7) | > 32 | 603 | 0 (0.0) | 511 (84.7) | > 32 |
| MDR P. aeruginosa | ||||||||
| Imipenem/relebactam | 229 | 134 (58.5) | 72 (31.4) | > 32 | 606 | 415 (68.5) | 129 (21.3) | 32 |
| Imipenem | 229 | 48 (21.0) | 169 (73.8) | > 32 | 606 | 162 (26.7) | 400 (66.0) | > 32 |
Abbreviations: IAIs, intra-abdominal infections; ICU, intensive care unit; MDR, multidrug-resistance; MIC, minimum inhibitory concentrations; N, number; R, resistant rate; RTIs, respiratory tract infections; S, susceptible rate; UTIs, urinary tract infections.
The majority of P. aeruginosa isolates were obtained from RTIs (65.6%), with 26.5% collected in ICUs and 73.5% in non-ICUs, followed by IAIs (21.5%) with 20.7% collected in ICUs and 79.3% in non-ICUs, and UTIs (12.9%) of which 11.9% were collected in ICUs and 88.1% in non-ICUs. Since P. aeruginosa has been found to be the second most common organism causing health care-associated pneumonia infections, the high number of RTI isolates is in line with the literature.8 Imipenem susceptibilities of ICU (59.5.%) and non-ICU (60.7%) isolates were similar for IAIs, but isolates collected in ICUs were less susceptible to imipenem compared to non-ICU isolates for RTIs (43.6% vs 53.0%) and UTIs (55.2% vs 77.1%) (Supplementary Table 4). The RTI and UTI data are in line with previously published literature in which the rate of carbapenem resistance was reported to be significantly higher in ICU vs non-ICU settings.35 However, the non-ICU IAI isolates had a higher rate of imipenem susceptibility compared to RTI and UTI isolates, which indicated that imipenem non-susceptibilities in IAIs have spread into non-ICU departments within hospitals.
Restoration of imipenem susceptibility by relebactam in imipenem non-susceptible P. aeruginosa isolates was similar in RTI isolates from ICUs (62.2%) and non-ICUs (64.0%), but essentially lower in ICU compared to non-ICU isolates collected from IAIs (50.0% vs 77.8%) and UTIs (30.8% vs 61.2%), a trend which was also exhibited for MDR P. aeruginosa isolates with similar imipenem restoration rates (Supplementary Table 4). These data indicated that P. aeruginosa isolates from RTIs, though with a higher imipenem resistance rate in ICUs, could be restored by relebactam to similar levels as for isolates from non-ICUs, which was not the case for UTI and IAI P. aeruginosa isolates. These findings may indicate that different carbapenem resistance mechanism distributions occurred in various organs and in ICU vs non-ICU infections and that imipenem resistance in ICU departments is caused by mechanisms other than Amber A or C β-lactamases.36,37
However, the similar rates of imipenem susceptibility restoration in imipenem non-susceptible and MDR P. aeruginosa isolates suggest that relebactam can be used also for MDR P. aeruginosa strains with similar efficacy.
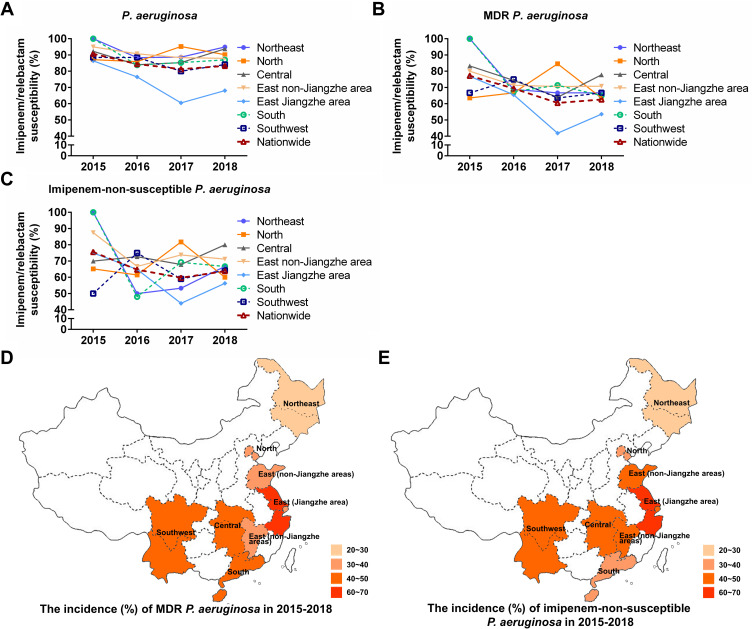
Next, we investigated the susceptibility rates of P. aeruginosa, MDR P. aeruginosa and imipenem-non-susceptible P. aeruginosa to imipenem/relebactam in different regions of China between 2015 and 2018. The susceptibility rate of imipenem/relebactam against P. aeruginosa has slightly decreased since 2015 but was maintained above 80% across all regions, except for the east Jiangzhe area (Figure 3A). There were more annual fluctuations and local differences of imipenem/relebactam susceptibilities in MDR and imipenem-non-susceptible P. aeruginosa isolates during the years 2015 to 2018, but most MDR P. aeruginosa isolates were susceptible to imipenem/relebactam in a range of > 60% – < 80%, with the exception of the east Jiangzhe area with essentially lower susceptibilities in 2017 and 2018 (Figure 3B). For imipenem-non-susceptible P. aeruginosa isolates, susceptibility rates to imipenem/relebactam were in the range of about ≥ 50% – < 80% between 2015 and 2018, with again the lowest susceptibilities in the east Jiangzhe area in 2017 (< 50%) and 2018 (Figure 3C). Low susceptibility rates of MDR and imipenem-non-susceptible P. aeruginosa isolates in the east Jiangzhe area correlated also with the highest incidences in this area (Figure 3D and E). Relebactam produced less improvement to the imipenem susceptibility in MDR P. aeruginosa isolates especially in the east Jiangzhe region where the MDR incidence rate was the highest. The reason for regional susceptibility differences of imipenem/relebactam needs further study, which should focus on resistance mechanisms in MDR isolates especially in areas with high resistance rates. However, relebactam cannot restore imipenem activity in strains producing metallo-β-lactamases,17,38 which has also been found for Chinese P. aeruginosa isolates36,37 and might explain local differences of relebactam efficacy in imipenem-no-susceptible P. aeruginosa strains.
Figure 3.
Changes in the susceptibility of (A) P. aeruginosa, (B) MDR P. aeruginosa and (C) imipenem-non-susceptible P. aeruginosa to imipenem/relebactam over time in different regions of China (2015, 2016, 2017, 2018). Country map to show the incidence (%) of (D) MDR P. aeruginosa and (E) imipenem-non-susceptible P. aeruginosa in different regions of China from 2015 to 2018.
Abbreviation: MDR, multidrug-resistance.
One major strength of the present study was the large sample size but one weakness is the inherent limitation that no molecular analyses were carried out.
Conclusions
In this study we found that relebactam was still an effective antimicrobial agent for the treatment of infections caused by P. aeruginosa, with susceptibility rates of 84.2% to imipenem/relebactam compared to 55.7% with solely imipenem. However, our study also echoes that carbapenem-resistant P. aeruginosa is a serious threat to public health in China, since relebactam could restore the rate of imipenem susceptibility by only 64.4%. The susceptibilities of P. aeruginosa isolates to amikacin were 89.7% and to colistin 94.9%, and to all other antibiotics < 65%. For A. baumannii, relebactam had no effect on MDR or imipenem non-susceptible isolates. Except for amikacin (32.7%) and colistin (96.2%), the percent susceptibility of A. baumannii to other antibiotics was < 22.2%.
Acknowledgments
The authors thank Aaron Johnson of IHMA, Inc. for his contributions to the conception and design, collection and assembly of data, provision of study materials and for obtaining funding, administrative, technical and logistic support. Medical writing and editorial assistance were provided by Shanghai BIOMED Science Technology (Shanghai, China) through funding provided by MSD China. The authors were solely responsible for the conception and implementation of this study and for writing the manuscript.
Funding Statement
This study was sponsored by funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Further support was provided by Beijing Key Clinical Specialty for Laboratory Medicine-Excellent Project (grant number ZK201000) and the National Key Research and Development Program of China (grant numbers 2018YFC1200100, 2018YFC1200105).
Abbreviations
ATCC, American Type Culture Collection; CHINET, China Antimicrobial Surveillance Network; CLSI, Clinical and Laboratory Standards Institute; IAIs, intra-abdominal infections; ICUs, intensive care units; KPC, Klebsiella pneumoniae carbapenemase; MICs, minimum inhibitory concentrations; MDR, multidrug-resistance; OXA, oxacillinase; QC, quality control; RTIs, respiratory tract infections; SMART, Study for Monitoring Antimicrobial Resistance Trends; UTIs, urinary tract infections.
Data Sharing Statement
The SMART database is not public and is only accessible to SMART investigators, but the data that support the findings of this study are directly available from MSD China or from the first author Hui Zhang upon reasonable request and with permission of MSD China.
Ethics Approval and Consent to Participate
The protocol was reviewed by the human research ethics committee of the Institutional Review Board (IRB) of the Peking Union Medical College Hospital. The project falls under the category observational study and all bacterial strains were from residual samples used in clinical diagnosis or were strains from their subcultures. This project did not involve any patient information nor did it affect the normal diagnosis and treatment of patients, and after consultation with the IRB, formal ethical approval was granted and written patient consent was not required (Ethics Approval Number: S-K238).
Author Contributions
All authors made substantial contributions to the conception and design of the study, acquisition of data, or analysis and interpretation of data, took part in drafting the manuscript or critically revising it for important intellectual content, agreed to submit it to the current journal, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Disclosure
Weijuan Zhang is an employee of MSD China. The other authors declare that they have no competing interests.
References
- 1.Rice LB. Progress and challenges in implementing the research on ESKAPE pathogens. Infect Control Hosp Epidemiol. 2010;31(Suppl 1):S7–10. [DOI] [PubMed] [Google Scholar]
- 2.Antunes LCS, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71(3):292–301. doi: 10.1111/2049-632X.12125 [DOI] [PubMed] [Google Scholar]
- 3.Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis. 2017;36(11):1999–2006. doi: 10.1007/s10096-016-2703-z [DOI] [PubMed] [Google Scholar]
- 4.Chopra T, Marchaim D, Awali RA, et al. Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother. 2013;57(12):6270–6275. doi: 10.1128/AAC.01520-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler DA, Biagi M, Tan X, Qasmieh S, Bulman ZP, Wenzler E. Multidrug resistant acinetobacter baumannii: resistance by any other name would still be hard to treat. Curr Infect Dis Rep. 2019;21(12):46. doi: 10.1007/s11908-019-0706-5 [DOI] [PubMed] [Google Scholar]
- 6.Gao L, Lyu Y, Li Y. Trends in drug resistance of acinetobacter baumannii over a 10-year period: nationwide data from the china surveillance of antimicrobial resistance program. Chin Med J. 2017;130(6):659–664. doi: 10.4103/0366-6999.201601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–1301. doi: 10.1017/ice.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 10.Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49(1):86–98. doi: 10.1177/1060028014556652 [DOI] [PubMed] [Google Scholar]
- 11.Olsen I. New promising β-lactamase inhibitors for clinical use. Eur J Clin Microbiol Infect Dis. 2015;34(7):1303–1308. doi: 10.1007/s10096-015-2375-0 [DOI] [PubMed] [Google Scholar]
- 12.Drawz SM, Papp-Wallace KM, Bonomo RA. New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother. 2014;58(4):1835–1846. doi: 10.1128/AAC.00826-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis. 2020;70(9):1799–1808. doi: 10.1093/cid/ciz530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Titov I, Wunderink RG, Roquilly A. et al. A randomized, double-blind, multicenter trial comparing efficacy and safety of imipenem/cilastatin/relebactam versus piperacillin/tazobactam in adults with hospital-acquired or ventilator-associated bacterial pneumonia (RESTORE-IMI 2 Study). Clin Infect Dis;2020. ciaa803. doi: 10.1093/cid/ciaa803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of Imipenem with relebactam against gram-negative pathogens from New York City. Antimicrob Agents Chemother. 2015;59(8):5029–5031. doi: 10.1128/AAC.00830-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young K, Raghoobar SL, Hairston NN. In vitro Activity of the Class a and C b-Lactamase Inhibitor MK-7655. Posters of the Fiftieth Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, USA, 2010. Poster F1-2139. Washington, DC, USA: American Society for Microbiology; 2010. [Google Scholar]
- 17.Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 2013;68(10):2286–2290. [DOI] [PubMed] [Google Scholar]
- 18.Lob S, Hackel M, Kazmierczak K, et al. In vitro activity of imipenem-relebactam against gram-negative bacilli isolated from patients with lower respiratory tract infections in the United States in 2015: results from the SMART global surveillance program. Diagn Microbiol Infect Dis. 2017;88:171–176. doi: 10.1016/j.diagmicrobio.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 19.Karlowsky JA, Lob SH, Kazmierczak KM, Young K, Motyl MR, Sahm DF. In vitro activity of imipenem-relebactam against clinical isolates of gram-negative bacilli isolated in hospital laboratories in the united states as part of the SMART 2016 program. Antimicrob Agents Chemother. 2018;62(7):e00169–00118. doi: 10.1128/AAC.00169-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lob SH, Hackel MA, Kazmierczak KM, et al. In Vitro Activity of Imipenem-Relebactam against Gram-Negative ESKAPE Pathogens Isolated by Clinical Laboratories in the United States in 2015 (Results from the SMART Global Surveillance Program). Antimicrob Agents Chemother. 2017;61(6):e02209–02216. doi: 10.1128/AAC.02209-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lob SH, Karlowsky JA, Young K, et al. Activity of imipenem/relebactam against MDR Pseudomonas aeruginosa in Europe: SMART 2015–2017. J Antimicrob Chemother. 2019;74(8):2284–2288. doi: 10.1093/jac/dkz191 [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 28th. CLSI M100. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 23.U. S. Food & Drug A. Antibacterial Susceptibility Test Interpretive Criteria. US Food and Drug Administration; 2020. [Google Scholar]
- 24.Xu Y, Huo R, Yan Z, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Chin J Nosocomiol. 2017;27(1):231–240. [DOI] [PubMed] [Google Scholar]
- 25.Karlowsky JA, Lob SH, Young K, Motyl MR, Sahm DF. Activity of imipenem/relebactam against Pseudomonas aeruginosa with antimicrobial-resistant phenotypes from seven global regions: SMART 2015–2016. J Glob Antimicrob Resist. 2018;15:140–147. doi: 10.1016/j.jgar.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 26.Yin D, Wu S, Yang Y, et al. Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the in vitro activities of ceftazidime-avibactam and ceftolozane-tazobactam against clinical isolates of enterobacteriaceae and pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019;63(4). doi: 10.1128/AAC.02431-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlowsky JA, Lob SH, Kazmierczak KM, et al. In vitro activity of imipenem/relebactam against Gram-negative ESKAPE pathogens isolated in 17 European countries: 2015 SMART surveillance programme. J Antimicrob Chemother. 2018;73(7):1872–1879. doi: 10.1093/jac/dky107 [DOI] [PubMed] [Google Scholar]
- 28.Han L, Lei J, Xu J, Han S. blaOXA-23-like and blaTEM rather than blaOXA-51-like contributed to a high level of carbapenem resistance in Acinetobacter baumannii strains from a teaching hospital in Xi’an, China. Medicine. 2017;96(48):e8965. doi: 10.1097/MD.0000000000008965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Beltrán J, Cabot G, Valencia EY, et al. N-acetylcysteine selectively antagonizes the activity of imipenem in Pseudomonas aeruginosa by an OprD-mediated mechanism. Antimicrob Agents Chemother. 2015;59(6):3246–3251. doi: 10.1128/AAC.00017-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect. 2006;12(9):826–836. doi: 10.1111/j.1469-0691.2006.01456.x [DOI] [PubMed] [Google Scholar]
- 31.Seward RJ, Lambert T, Towner KJ. Molecular epidemiology of aminoglycoside resistance in Acinetobacter spp. J Med Microbiol. 1998;47(5):455–462. doi: 10.1099/00222615-47-5-455 [DOI] [PubMed] [Google Scholar]
- 32.Falagas ME, Kasiakou SK, Saravolatz LD. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40(9):1333–1341. doi: 10.1086/429323 [DOI] [PubMed] [Google Scholar]
- 33.Tosi M, Roat E, De Biasi S, et al. Multidrug resistant bacteria in critically ill patients: a step further antibiotic therapy. J Emerg Crit Care Med. 2018;2:103. doi: 10.21037/jeccm.2018.11.08 [DOI] [Google Scholar]
- 34.Wieland K, Chhatwal P, Vonberg RP. Nosocomial outbreaks caused by Acinetobacter baumannii and Pseudomonas aeruginosa: results of a systematic review. Am J Infect Control. 2018;46(6):643–648. doi: 10.1016/j.ajic.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 35.McCann E, Srinivasan A, DeRyke CA, et al. Carbapenem-nonsusceptible gram-negative pathogens in ICU and non-ICU settings in us hospitals in 2017: a multicenter study. Open Forum Infect Dis. 2018;5(10):ofy241. doi: 10.1093/ofid/ofy241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Niu H, Hu T, et al. High expression of metallo-β-lactamase contributed to the resistance to carbapenem in clinical isolates of pseudomonas aeruginosa from Baotou, China. Infect Drug Resist. 2020;13:35–43. doi: 10.2147/IDR.S233987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan X, Wu Y, Xiao M, et al. Diverse Genetic background of multidrug-resistant pseudomonas aeruginosa from mainland china and emergence of an extensively drug-resistant ST292 clone in kunming. Sci Rep. 2016;6(1):26522. doi: 10.1038/srep26522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tselepis L, Langley GW, Aboklaish AF, et al. In vitro efficacy of imipenem-relebactam and cefepime-AAI101 against a global collection of ESBL-positive and carbapenemase-producing Enterobacteriaceae. Int J Antimicrob Agents. 2020;56(1):105925. doi: 10.1016/j.ijantimicag.2020.105925 [DOI] [PubMed] [Google Scholar]