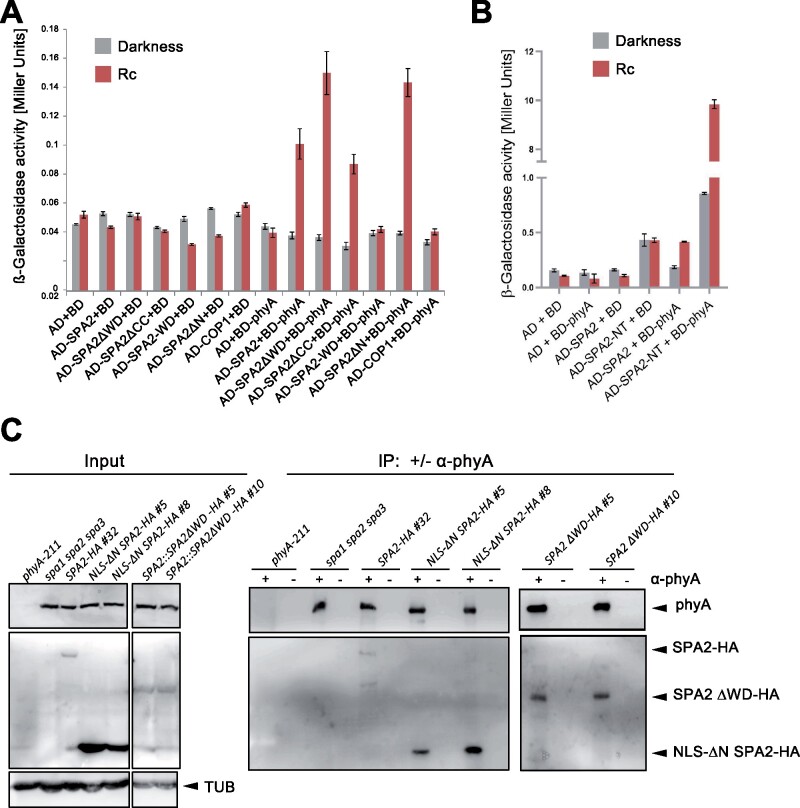
Figure 7.
The N-terminal and WD-repeat domains of SPA2 are not necessary for the SPA2-phyA interaction in yeast two-hybrid and in vivo. A and B, Yeast two-hybrid assays expressing the indicated proteins. Yeast cells were grown in darkness or in 1 μmol m−2 s−1 continuous red light (Rc). Error bars indicate the sem. C, Co-immunoprecipitation of SPA2-HA and SPA2-HA deletion proteins by phyA. Seedlings of the indicated genotypes were grown in darkness for 4 d, incubated in MG132 for 3 h to prevent SPA2-HA degradation and then transferred to 5 μmol m−2s−1 Rc for 10 min to allow nuclear accumulation of phyA. After further 10 min in darkness, phyA was immunoprecipitated using protein-A beads with (+) or without (−) a coupled phyA antibody. Co-immunoprecipitated SPA2-HA or SPA2-HA deletion proteins were detected using an α-HA antibody. phyA was detected using an α-phyA antibody. Tubulin (TUB) served as a loading control for the input samples. All input samples were run on the same gel with one lane removed. The IP samples were run on two separate gels.